Global temporal changes in the proportion of children with advanced disease at the start of combination antiretroviral therapy in an era of changing criteria for treatment initiation
- PMID: 30614622
- PMCID: PMC6275813
- DOI: 10.1002/jia2.25200
Global temporal changes in the proportion of children with advanced disease at the start of combination antiretroviral therapy in an era of changing criteria for treatment initiation
Abstract
Introduction: The CD4 cell count and percent at initiation of combination antiretroviral therapy (cART) are measures of advanced HIV disease and thus are important indicators of programme performance for children living with HIV. In particular, World Health Organization (WHO) 2017 guidelines on advanced HIV disease noted that >80% of children aged <5 years started cART with WHO Stage 3 or 4 disease or severe immune suppression. We compared temporal trends in CD4 measures at cART start in children from low-, middle- and high-income countries, and examined the effect of WHO treatment initiation guidelines on reducing the proportion of children initiating cART with advanced disease.
Methods: We included children aged <16 years from the International Epidemiology Databases to Evaluate acquired immunodeficiency syndrome (AIDS) (IeDEA) Collaboration (Caribbean, Central and South America, Asia-Pacific, and West, Central, East and Southern Africa), the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE), the North American Pediatric HIV/AIDS Cohort Study (PHACS) and International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 219C study. Severe immunodeficiency was defined using WHO guidelines. We used generalized weighted additive mixed effect models to analyse temporal trends in CD4 measurements and piecewise regression to examine the impact of 2006 and 2010 WHO cART initiation guidelines.
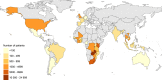
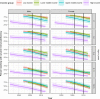
Results: We included 52,153 children from fourteen low-, eight lower middle-, five upper middle- and five high-income countries. From 2004 to 2013, the estimated percentage of children starting cART with severe immunodeficiency declined from 70% to 42% (low-income), 67% to 64% (lower middle-income) and 61% to 43% (upper middle-income countries). In high-income countries, severe immunodeficiency at cART initiation declined from 45% (1996) to 14% (2012). There were annual decreases in the percentage of children with severe immunodeficiency at cART initiation after the WHO guidelines revisions in 2006 (low-, lower middle- and upper middle-income countries) and 2010 (all countries).
Conclusions: By 2013, less than half of children initiating cART had severe immunodeficiency worldwide. WHO treatment initiation guidelines have contributed to reducing the proportion of children and adolescents starting cART with advanced disease. However, considerable global inequity remains, in 2013, >40% of children in low- and middle-income countries started cART with severe immunodeficiency compared to <20% in high-income countries.
Keywords: Asia; CD4 cell count; Caribbean; Central and South America; Europe; North America; WHO guidelines; advanced HIV disease; antiretroviral therapy; sub-Saharan Africa.
© 2018 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures

Similar articles
-
Global Trends in CD4 Cell Count at the Start of Antiretroviral Therapy: Collaborative Study of Treatment Programs.Clin Infect Dis. 2018 Mar 5;66(6):893-903. doi: 10.1093/cid/cix915. Clin Infect Dis. 2018. PMID: 29373672 Free PMC article.
-
Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries.J Acquir Immune Defic Syndr. 2015 Jan 1;68(1):62-72. doi: 10.1097/QAI.0000000000000380. J Acquir Immune Defic Syndr. 2015. PMID: 25501345 Free PMC article.
-
Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-Saharan Africa, Asia-Pacific, and central and South America.J Int AIDS Soc. 2012 Jan 30;15(1):5. doi: 10.1186/1758-2652-15-5. J Int AIDS Soc. 2012. PMID: 22289654 Free PMC article.
-
Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies.Lancet. 2009 Apr 18;373(9672):1352-63. doi: 10.1016/S0140-6736(09)60612-7. Epub 2009 Apr 8. Lancet. 2009. PMID: 19361855 Free PMC article. Review.
-
[National consensus document by GESIDA/National Aids Plan on antiretroviral treatment in adults infected by the human immunodeficiency virus (January 2011 update)].Enferm Infecc Microbiol Clin. 2011 Mar;29(3):209.e1-103. doi: 10.1016/j.eimc.2010.12.004. Enferm Infecc Microbiol Clin. 2011. PMID: 21388714 Spanish.
Cited by
-
Global Trends in CD4 Measurement and Immunosuppression at ART Initiation Among Children With HIV.Pediatr Infect Dis J. 2025 Apr 4:10.1097/INF.0000000000004819. doi: 10.1097/INF.0000000000004819. Online ahead of print. Pediatr Infect Dis J. 2025. PMID: 40233289 Free PMC article.
-
The changing characteristics of a cohort of children and adolescents living with HIV at antiretroviral therapy initiation in Asia.PLoS One. 2023 Sep 14;18(9):e0291523. doi: 10.1371/journal.pone.0291523. eCollection 2023. PLoS One. 2023. PMID: 37708128 Free PMC article.
-
International epidemiology databases to evaluate AIDS (IeDEA) in sub-Saharan Africa, 2012-2019.BMJ Open. 2020 May 15;10(5):e035246. doi: 10.1136/bmjopen-2019-035246. BMJ Open. 2020. PMID: 32414825 Free PMC article.
-
The Enduring Burden of Advanced Human Immunodeficiency Virus Disease.Clin Infect Dis. 2020 Dec 3;71(9):2500-2501. doi: 10.1093/cid/ciaa265. Clin Infect Dis. 2020. PMID: 32369101 Free PMC article. No abstract available.
-
Effectiveness of symptom-based diagnostic HIV testing versus targeted and blanket provider-initiated testing and counseling among children and adolescents in Cameroon.PLoS One. 2019 May 6;14(5):e0214251. doi: 10.1371/journal.pone.0214251. eCollection 2019. PLoS One. 2019. PMID: 31059507 Free PMC article.
References
-
- WHO . Antiretroviral therapy of HIV infection in infants and children: towards universal access. 2006. [cited 2007 Feb 09]. Available from: http://www.who.int/hiv/pub/paediatric/infants/en/index.html - PubMed
-
- WHO . Antiretroviral therapy for HIV infection in infants and children: Towards universal access. Recommendations for a public health approach: 2010 revision. 2010. [cited 2010 Oct 19]. Available from: http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html - PubMed
-
- WHO . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Summary of key features and recommendations. 2013. [cited 2013 Nov 10]. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/short_summary/en/index.html
-
- World Health Organization . Guideline on when to start antiretroviral therapy and on pre‐exposure prophylaxis for HIV. 2015. [cited 2015 Sep 30]. Available from: http://who.int/hiv/en/ - PubMed
-
- Cross Continents Collaboration for Kids (3Cs4kids) Analysis and Writing Committee . Markers for predicting mortality in untreated HIV‐infected children in resource‐limited settings: a meta‐analysis. AIDS. 2008;22:97–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HD052104/HD/NICHD NIH HHS/United States
- U01 AI096299/AI/NIAID NIH HHS/United States
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- N01-HD-8-0001/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/International
- U01 AI069923/AI/NIAID NIH HHS/United States
- MC_UU_12023/26/MRC_/Medical Research Council/United Kingdom
- U01 AI068616/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- U01 AI069919/AI/NIAID NIH HHS/United States
- U01 AI069924/AI/NIAID NIH HHS/United States
- U01 AI069911/AI/NIAID NIH HHS/United States
- N01-HD-3-3345/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/International
- U01 AI069907/AI/NIAID NIH HHS/United States
- U01 HD052102/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

