Improved Differential Diagnosis of Alzheimer's Disease by Integrating ELISA and Mass Spectrometry-Based Cerebrospinal Fluid Biomarkers
- PMID: 30614806
- PMCID: PMC6398544
- DOI: 10.3233/JAD-180855
Improved Differential Diagnosis of Alzheimer's Disease by Integrating ELISA and Mass Spectrometry-Based Cerebrospinal Fluid Biomarkers
Abstract
Background: Alzheimer's disease (AD) is diagnosed based on a clinical evaluation as well as analyses of classical biomarkers: Aβ42, total tau (t-tau), and phosphorylated tau (p-tau) in cerebrospinal fluid (CSF). Although the sensitivities and specificities of the classical biomarkers are fairly good for detection of AD, there is still a need to develop novel biochemical markers for early detection of AD.
Objective: We explored if integration of novel proteins with classical biomarkers in CSF can better discriminate AD from non-AD subjects.
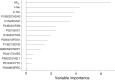
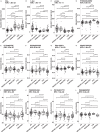
Methods: We applied ELISA, mass spectrometry, and multivariate modeling to investigate classical biomarkers and the CSF proteome in subjects (n = 206) with 76 AD patients, 74 mild cognitive impairment (MCI) patients, 11 frontotemporal dementia (FTD) patients, and 45 non-dementia controls. The MCI patients were followed for 4-9 years and 21 of these converted to AD, whereas 53 remained stable.
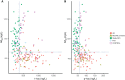
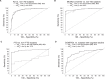
Results: By combining classical CSF biomarkers with twelve novel markers, the area of the ROC curves (AUROCS) of distinguishing AD and MCI/AD converters from non-AD were 93% and 96%, respectively. The FTDs and non-dementia controls were identified versus all other groups with AUROCS of 96% and 87%, respectively.
Conclusions: Integration of new and classical CSF biomarkers in a model-based approach can improve the identification of AD, FTD, and non-dementia control subjects.
Keywords: Alzheimer’s disease; ELISA; cerebrospinal fluid; mass spectrometry; mild cognitive impairment; proteomics.
Figures
References
-
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol 5, 228–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

