Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen
- PMID: 30614873
- PMCID: PMC7065840
- DOI: 10.1097/SLA.0000000000003053
Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen
Abstract
Objective: The objective of this work was to causatively link biofilm properties of bacterial infection to specific pathogenic mechanisms in wound healing.
Background: Staphylococcus aureus is one of the four most prevalent bacterial species identified in chronic wounds. Causatively linking wound pathology to biofilm properties of bacterial infection is challenging. Thus, isogenic mutant stains of S. aureus with varying degree of biofilm formation ability was studied in an established preclinical porcine model of wound biofilm infection.
Methods: Isogenic mutant strains of S. aureus with varying degree (ΔrexB > USA300 > ΔsarA) of biofilm-forming ability were used to infect full-thickness porcine cutaneous wounds.
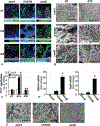
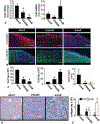
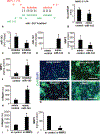
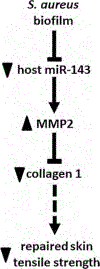
Results: Compared with that of ΔsarA infection, wound biofilm burden was significantly higher in response to ΔrexB or USA300 infection. Biofilm infection caused degradation of cutaneous collagen, specifically collagen 1 (Col1), with ΔrexB being most pathogenic in that regard. Biofilm infection of the wound repressed wound-edge miR-143 causing upregulation of its downstream target gene matrix metalloproteinase-2. Pathogenic rise of collagenolytic matrix metalloproteinase-2 in biofilm-infected wound-edge tissue sharply decreased collagen 1/collagen 3 ratio compromising the biomechanical properties of the repaired skin. Tensile strength of the biofilm infected skin was compromised supporting the notion that healed wounds with a history of biofilm infection are likely to recur.
Conclusion: This study provides maiden evidence that chronic S. aureus biofilm infection in wounds results in impaired granulation tissue collagen leading to compromised wound tissue biomechanics. Clinically, such compromise in tissue repair is likely to increase wound recidivism.
Conflict of interest statement
The authors report no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

