Immunometabolic Alterations by HPV Infection: New Dimensions to Head and Neck Cancer Disparity
- PMID: 30615137
- PMCID: PMC6410958
- DOI: 10.1093/jnci/djy207
Immunometabolic Alterations by HPV Infection: New Dimensions to Head and Neck Cancer Disparity
Abstract
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer, with high morbidity and mortality. Racial disparity in HNSCC is observed between African Americans (AAs) and whites, effecting both overall and 5-year survival, with worse prognosis for AAs. In addition to socio-economic status and demographic factors, many epidemiological studies have also identified factors including coexisting human papillomavirus (HPV) infection, primary tumor location, and a variety of somatic mutations that contribute to the prognostic incongruities in HNSCC patients among AAs and whites. Recent research also suggests HPV-induced dysregulation of tumor metabolism and immune microenvironment as the major regulators of HNSCC patient prognosis. Outcomes of several preclinical and clinical studies on targeted therapeutics warrant the need to elucidate the inherent mechanistic and population-based disparities underlying patient responses. This review systematically reports the underlying reasons for inconsistency in disease prognosis and therapy responses among HNSCC patients from different racial populations. The focus of this review is twofold: aside from discussing the causes of racial disparity, we also seek to identify the consequences of such disparity in terms of HPV infection and its associated mutational, metabolic, and immune landscapes. Considering the clinical impact of differential patient outcomes among AA and white populations, understanding the underlying cause of this disparity may pave the way for novel precision therapy for HNSCC.
Published by Oxford University Press 2019.
Figures

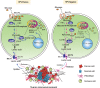
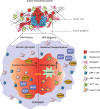
References
-
- Berman TA, Schiller JT.. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123(12):2219–2229. - PubMed
-
- De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

