Induction Chemotherapy Response as a Guide for Treatment Optimization in Sinonasal Undifferentiated Carcinoma
- PMID: 30615549
- PMCID: PMC6380524
- DOI: 10.1200/JCO.18.00353
Induction Chemotherapy Response as a Guide for Treatment Optimization in Sinonasal Undifferentiated Carcinoma
Abstract
Purpose: Multimodal therapy is a well-established approach for the treatment of sinonasal undifferentiated carcinoma (SNUC); however, the optimal sequence of the various treatments modalities is yet to be determined. This study aimed to assess the role of induction chemotherapy (IC) in guiding definitive therapy in patients with SNUC.
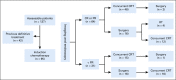
Methods: Ninety-five previously untreated patients diagnosed with SNUC and treated between 2001 and 2018 at The University of Texas MD Anderson Cancer Center were included in the analysis. Patients were treated with curative intent and received IC before definitive locoregional therapy. The primary end point was disease-specific survival (DSS). Secondary end points included overall and disease-free survival, disease recurrence, and organ preservation.
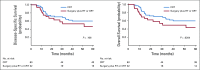
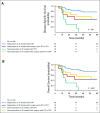
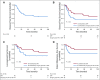
Results: A total of 95 treatment-naïve patients were included in the analysis. For the entire cohort, the 5-years DSS probability was 59% (95% CI, 53% to 66%). In patients who had partial or complete response to IC, the 5-year DSS probabilities were 81% (95% CI, 69% to 88%) after treatment with definitive concurrent chemoradiotherapy (CRT) after IC and 54% (95% CI, 44% to 61%) after definitive surgery and postoperative radiotherapy or CRT after IC (log-rank P = .001). In patients who did not experience at least a partial response to IC, the 5-year DSS probabilities were 0% (95% CI, 0% to 4%) in patients who were treated with concurrent CRT after IC and 39% (95% CI, 30% to 46%) in patients who were treated with surgery plus radiotherapy or CRT (adjusted hazard ratio of 5.68 [95% CI, 2.89 to 9.36]).
Conclusion: In patients who achieve a favorable response to IC, definitive CRT results in improved survival compared with those who undergo definitive surgery. In patients who do not achieve a favorable response to IC, surgery when feasible seems to provide a better chance of disease control and improved survival.
Figures

References
-
- Thompson LDR BD, Bishop JA.Neuroendocrine carcinomasinEl-Naggar AK, Chan JKC, Grandis JR, et al.(eds)WHO Classification of Head and Neck Tumors ed 4Lyon, France: IARC Press; 2017. pp21–23.
-
- Zielinski V, Laban S, Tribius S, et al. Management of sinonasal undifferentiated carcinoma with intracerebral invasion: Clinical experience at a single institution and review of the literature. Ear Nose Throat J. 2016;95:23–28. - PubMed
-
- Lopez F, Suárez V, Vivanco B, et al. Current management of sinonasal undifferentiated carcinoma. Rhinology. 2015;53:212–220. - PubMed
-
- Tanzler ED, Morris CG, Orlando CA, et al. Management of sinonasal undifferentiated carcinoma. Head Neck. 2008;30:595–599. - PubMed
-
- Chen AM, Daly ME, El-Sayed I, et al. Patterns of failure after combined-modality approaches incorporating radiotherapy for sinonasal undifferentiated carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2008;70:338–343. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

