A transient helix in the disordered region of dynein light intermediate chain links the motor to structurally diverse adaptors for cargo transport
- PMID: 30615611
- PMCID: PMC6336354
- DOI: 10.1371/journal.pbio.3000100
A transient helix in the disordered region of dynein light intermediate chain links the motor to structurally diverse adaptors for cargo transport
Abstract
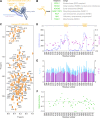
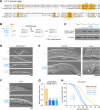
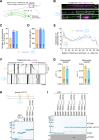
All animal cells use the motor cytoplasmic dynein 1 (dynein) to transport diverse cargo toward microtubule minus ends and to organize and position microtubule arrays such as the mitotic spindle. Cargo-specific adaptors engage with dynein to recruit and activate the motor, but the molecular mechanisms remain incompletely understood. Here, we use structural and dynamic nuclear magnetic resonance (NMR) analysis to demonstrate that the C-terminal region of human dynein light intermediate chain 1 (LIC1) is intrinsically disordered and contains two short conserved segments with helical propensity. NMR titration experiments reveal that the first helical segment (helix 1) constitutes the main interaction site for the adaptors Spindly (SPDL1), bicaudal D homolog 2 (BICD2), and Hook homolog 3 (HOOK3). In vitro binding assays show that helix 1, but not helix 2, is essential in both LIC1 and LIC2 for binding to SPDL1, BICD2, HOOK3, RAB-interacting lysosomal protein (RILP), RAB11 family-interacting protein 3 (RAB11FIP3), ninein (NIN), and trafficking kinesin-binding protein 1 (TRAK1). Helix 1 is sufficient to bind RILP, whereas other adaptors require additional segments preceding helix 1 for efficient binding. Point mutations in the C-terminal helix 1 of Caenorhabditis elegans LIC, introduced by genome editing, severely affect development, locomotion, and life span of the animal and disrupt the distribution and transport kinetics of membrane cargo in axons of mechanosensory neurons, identical to what is observed when the entire LIC C-terminal region is deleted. Deletion of the C-terminal helix 2 delays dynein-dependent spindle positioning in the one-cell embryo but overall does not significantly perturb dynein function. We conclude that helix 1 in the intrinsically disordered region of LIC provides a conserved link between dynein and structurally diverse cargo adaptor families that is critical for dynein function in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Dynein light intermediate chains as pivotal determinants of dynein multifunctionality.J Cell Sci. 2021 May 15;134(10):jcs254870. doi: 10.1242/jcs.254870. Epub 2021 May 20. J Cell Sci. 2021. PMID: 34014309
-
Recruitment of dynein to late endosomes and lysosomes through light intermediate chains.Mol Biol Cell. 2011 Feb 15;22(4):467-77. doi: 10.1091/mbc.E10-02-0129. Epub 2010 Dec 17. Mol Biol Cell. 2011. PMID: 21169557 Free PMC article.
-
A conserved interaction of the dynein light intermediate chain with dynein-dynactin effectors necessary for processivity.Nat Commun. 2018 Mar 7;9(1):986. doi: 10.1038/s41467-018-03412-8. Nat Commun. 2018. PMID: 29515126 Free PMC article.
-
Dynein activators and adaptors at a glance.J Cell Sci. 2019 Mar 15;132(6):jcs227132. doi: 10.1242/jcs.227132. J Cell Sci. 2019. PMID: 30877148 Free PMC article. Review.
-
Regulators of the cytoplasmic dynein motor.Nat Rev Mol Cell Biol. 2009 Dec;10(12):854-65. doi: 10.1038/nrm2804. Nat Rev Mol Cell Biol. 2009. PMID: 19935668 Free PMC article. Review.
Cited by
-
A tunable LIC1-adaptor interaction modulates dynein activity in a cargo-specific manner.Nat Commun. 2020 Nov 10;11(1):5695. doi: 10.1038/s41467-020-19538-7. Nat Commun. 2020. PMID: 33173051 Free PMC article.
-
Sequential dynein effectors regulate axonal autophagosome motility in a maturation-dependent pathway.J Cell Biol. 2021 Jul 5;220(7):e202010179. doi: 10.1083/jcb.202010179. Epub 2021 May 20. J Cell Biol. 2021. PMID: 34014261 Free PMC article.
-
The KASH5 protein involved in meiotic chromosomal movements is a novel dynein activating adaptor.Elife. 2022 Jun 15;11:e78201. doi: 10.7554/eLife.78201. Elife. 2022. PMID: 35703493 Free PMC article.
-
CDR2 is a dynein adaptor recruited by kinectin to regulate ER sheet organization.J Cell Biol. 2025 Sep 1;224(9):e202411034. doi: 10.1083/jcb.202411034. Epub 2025 Jul 10. J Cell Biol. 2025. PMID: 40637585
-
Dynein directs prophase centrosome migration to control the stem cell division axis in the developing Caenorhabditis elegans epidermis.Genetics. 2024 Mar 6;226(3):iyae005. doi: 10.1093/genetics/iyae005. Genetics. 2024. PMID: 38213110 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

