Ex vivo biophysical characterization of a hydrogel-based artificial vitreous substitute
- PMID: 30615640
- PMCID: PMC6322733
- DOI: 10.1371/journal.pone.0209217
Ex vivo biophysical characterization of a hydrogel-based artificial vitreous substitute
Abstract
Purpose: To characterize the biophysical properties of an artificial vitreous body substitute (VBS), which consists of a biocompatible, cross-linked, hyaluronic acid (HA)-based hydrogel, by analysing the VBS's influence on intraocular pressure (IOP) and retinal integrity in distinct ex vivo eye models in order to evaluate the its potential for in vivo biocompatibility testing.
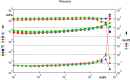
Methods: Pig eyes were obtained immediately postmortem, and VBS was injected after core-vitrectomy. IOP was followed for 24 h (n = 5). VBS influence on retinal integrity was investigated using isolated bovine retinas superfused with an oxygen saturated nutrient solution. An electroretinogram (ERG) was recorded on explanted bovine retinae using silver/silver chloride electrodes; after application of VBS for 2 min, a washout period of 70 min was employed. The percentage of a-and b-wave reduction at the end of the washout phase was compared to baseline values (n = 5). Data were calculated throughout as the mean and the standard deviation. qRT-PCR (Bax/Bcl-2-ratio, GFAP- and PGP9.5-levels) or western blot analysis was used to test for toxicity of Princess Volume after 24 h (and β-3 tubulin with GAPDH as a control gene). Significance was estimated by Student´s t-test; p ≤0.05 was considered to be statistically significant.
Results: The IOP increased non-significantly by 10% after 24 h. Short-term biocompatibility testing using isolated superfused bovine retinas showed neither significant reductions of the b-wave nor the a-wave amplitudes (b-wave reduction 14.2%, p>0.05; a-wave reduction 23.9%, p>0.05). qRT-PCR and western blot analysis did not reveal significant toxicity after 24 h.
Conclusions: The manufactured HA-based hydrogel showed highly favourable biophysical characteristics in the explored ex vivo models, justifying in vivo studies enabling the assessment of biocompatibility.
Conflict of interest statement
Parts of the experiments were financed by Croma Pharma GmbH, Austria. CH and CR are employed by Croma Pharma GmbH. There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures



References
-
- Spitzer MS, Januschowski K. [Aging and age-related changes of the vitreous body]. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2015. July;112(7):552, 4–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

