Characteristic, completion or matching timescales? An analysis of temporary boundaries in enzyme kinetics
- PMID: 30615881
- PMCID: PMC6612542
- DOI: 10.1016/j.jtbi.2019.01.005
Characteristic, completion or matching timescales? An analysis of temporary boundaries in enzyme kinetics
Abstract
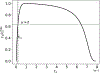
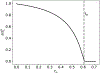
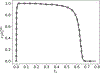
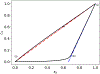
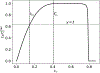
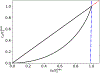
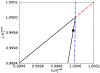
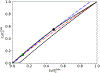
Scaling analysis exploiting timescale separation has been one of the most important techniques in the quantitative analysis of nonlinear dynamical systems in mathematical and theoretical biology. In the case of enzyme catalyzed reactions, it is often overlooked that the characteristic timescales used for the scaling the rate equations are not ideal for determining when concentrations and reaction rates reach their maximum values. In this work, we first illustrate this point by considering the classic example of the single-enzyme, single-substrate Michaelis-Menten reaction mechanism. We then extend this analysis to a more complicated reaction mechanism, the auxiliary enzyme reaction, in which a substrate is converted to product in two sequential enzyme-catalyzed reactions. In this case, depending on the ordering of the relevant timescales, several dynamic regimes can emerge. In addition to the characteristic timescales for these regimes, we derive matching timescales that determine (approximately) when the transitions from transient to quasi-steady-state kinetics occurs. The approach presented here is applicable to a wide range of singular perturbation problems in nonlinear dynamical systems.
Keywords: Chemical kinetics; Enzyme kinetics; Nonlinear dynamical systems; Perturbation methods; Slow and fast dynamics; Timescales.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures






References
-
- Berglund N, Gentz B, 2006. Noise-induced phenomena in slow-fast dynamical systems. Springer-Verlag London, Ltd., London.
-
- Bersani AM, Bersani E, Dell’Acqua G, Pedersen MG, 2015. New trends and perspectives in nonlinear intracellular dynamics: one century from Michaelis-Menten paper. Contin. Mech. Thermodyn 27, 659–684.
-
- Bersani AM, Dell’Acqua G, 2012. Is there anything left to say on enzyme kinetic constants and quasi-steady state approximation? J. Math. Chem 50, 335–344.
-
- Bertram R, Rubin JE, 2017. Multi-timescale systems and fast-slow analysis. Math. Biosci 287, 105–121. - PubMed
-
- Borghans JAM, Boer RJD, Segel LA, 1996. Extending the quasi-steady state approximation by changing variables. Bull. Math. Biol 58, 43–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

