Insulin receptor localization in the embryonic avian hypothalamus
- PMID: 30615976
- PMCID: PMC6435403
- DOI: 10.1016/j.neulet.2019.01.009
Insulin receptor localization in the embryonic avian hypothalamus
Abstract
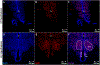
The hypothalamus is a brain region critical for the homeostatic regulation of appetite and energy expenditure. Hypothalamic neuronal activity that is altered during development can produce permanent physiological changes later in life. For example, circulating hormones such as insulin have been shown to influence hypothalamic neuronal projections, leading to altered metabolism in adult rodents. While insulin signaling in the post-hatch chicken has been shown to mirror that of mammals, the developmental role of insulin in the avian embryonic hypothalamus remains largely unexplored. Here we present the earliest known evidence for insulin receptor (InsR) expression in embryonic avian hypothalamic nuclei governing energy homeostasis. RT-PCR analysis reveals InsR mRNA in E8, E10, and E12 neurons while western blot data demonstrate protein expression in E12 avian whole brain and hypothalamic lysates. Immunohistochemical analysis of avian hypothalamic brain slices demonstrates a shift in InsR localization from paraventricular expression in E8 to a more defined concentration of InsR in developmental regions resembling the ventromedial hypothalamus (VMH) and arcuate nucleus (ARC) in E12 time points. In addition, InsR expression appears in a heterogeneous pattern, suggesting receptor localization to subpopulations of avian hypothalamic neurons as early as E8. With increasing evidence suggesting energy homeostasis pathways may be altered via the gestational environment, it is important to understand how insulin signaling may affect embryogenesis. Our research provides evidence for the earliest known embryonic expression of InsR protein in the avian hypothalamus and may suggest a developmental role for insulin signaling in the early patterning of metabolic pathways in the central nervous system.
Keywords: Avian embryo; Energy homeostasis; Hypothalamic development; Insulin receptor; Metabolism.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures



References
-
- Seoane-Collazo P, Ferno J, Gonzalez F, Dieguez C, Leis R, Nogueiras R, Lopez M, Hypothalamic-autonomic control of energy homeostasis, Endocrine. 50 (2015) 276–291. - PubMed
-
- Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D, Insulin in the brain: A hormonal regulator of energy balance, Endocr. Rev 13 (1992) 387–414. - PubMed
-
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW, Central nervous system control of food intake and body weight, Nature. 443 (2006) 289–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

