A basic solution to activate the cholinergic anti-inflammatory pathway via the mesothelium?
- PMID: 30616018
- PMCID: PMC6391187
- DOI: 10.1016/j.phrs.2019.01.007
A basic solution to activate the cholinergic anti-inflammatory pathway via the mesothelium?
Abstract
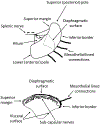
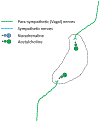
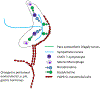
Much research now indicates that vagal nerve stimulation results in a systemic reduction in inflammatory cytokine production and an increase in anti-inflammatory cell populations that originates from the spleen. Termed the 'cholinergic anti-inflammatory pathway', therapeutic activation of this innate physiological response holds enormous promise for the treatment of inflammatory disease. Much controversy remains however, regarding the underlying physiological pathways mediating this response. This controversy is anchored in the fact that the vagal nerve itself does not innervate the spleen. Recent research from our own laboratory indicating that oral intake of sodium bicarbonate stimulates splenic anti-inflammatory pathways, and that this effect may require transmission of signals to the spleen through the mesothelium, provide new insight into the physiological pathways mediating the cholinergic anti-inflammatory pathway. In this review, we examine proposed models of the cholinergic anti-inflammatory pathway and attempt to frame our recent results in relation to these hypotheses. Following this discussion, we then provide an alternative model of the cholinergic anti-inflammatory pathway which is consistent both with our recent findings and the published literature. We then discuss experimental approaches that may be useful to delineate these hypotheses. We believe the outcome of these experiments will be critical in identifying the most appropriate methods to harness the therapeutic potential of the cholinergic anti-inflammatory pathway for the treatment of disease and may also shed light on the etiology of other pathologies, such as idiopathic fibrosis.
Keywords: Macrophage polarization; Perisplenitis; Sodium bicarbonate; Tumor-necrosis factor; Vagal nerve stimulation.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures




References
-
- Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

