Safety of Biologic Therapy in Older Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis
- PMID: 30616024
- PMCID: PMC6609492
- DOI: 10.1016/j.cgh.2018.12.032
Safety of Biologic Therapy in Older Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis
Abstract
Background & aims: Management of immune-mediated inflammatory diseases often requires lifelong immunosuppression. Increasing numbers of older patients have inflammatory diseases and are particularly vulnerable to risks of immune suppressive therapies-particularly infections and malignancies.
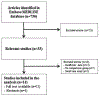
Methods: We systematically searched PubMed/Medline and Embase to identify eligible studies that examined the safety of biologic therapies in older patients with immune-mediated inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, psoriasis). Included studies provided information on patients who began receiving therapy with a biologic agent when they were older than 60 years and a control population (either younger users of biologics or older patients who did not use biologics). Information of on overall pooled rates of infections, malignancy, and mortality were extracted. A DerSimonian and Laird random effects model was used to calculate pooled odds ratios (ORs) and 95% CIs.
Results: Our meta-analysis included 14 unique studies that comprised 4719 older users of biologics, 13,305 younger users of biologics, and 3961 older patients who did not use biologics. The pooled prevalence of infections in older and younger users of biologics was 13% and 6% respectively, yielding a pooled random effects odds ratio of 2.28 (95% CI, 1.57-3.31). Older age was associated with a significant increase in risk of malignancy (OR, 3.07; 95% CI, 1.98-4.62) compared to younger age. Older users of biologics had a 3-fold increase in risk of infection compared to patients who did not use biologics (OR, 3.60; 95% CI, 1.62-8.01), but there were no significant differences in odds of malignancy (0.54, 95% CI, 0.28-1.05) or death (OR, 1.52; 95% CI, 0.44-5.28) compared to older patients who did not use biologics.
Conclusion: In a systematic review and meta-analysis of studies on the safety of biologic therapies in older patients with inflammatory diseases, we found that older users of biologic agents have an increased risk of infections compared with younger users or older patients who do not use biologics. Large, prospective cohort studies are needed to examine safety of biologic therapy in older patients with immune-mediated diseases.
Keywords: Anti-TNF Therapy; Autoimmune Disease; Drug; Elderly; IBD; Inflammatory Bowel Disease; Psoriasis; Rheumatoid Arthritis; Safety; Tumor Necrosis Factor.
Copyright © 2019 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States. Atlanta, Georgia, US: Centers for Disease Control and Prevention, 2011.
-
- US cancer statistics: 1999 – 2009 incidence and mortality web-based report. Volume http://www.cdc.gov/uscs. Atlanta, Georgia: U.S. Cancer Statistics Working Group, 2013.
-
- Siegel CA, Hur C, Korzenik JR, et al. Risks and benefits of infliximab for the treatment of Crohn’s disease. Clin Gastroenterol Hepatol 2006;4:1017–24; quiz 976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

