Patients with metastatic renal cell carcinoma who benefit from axitinib dose titration: analysis from a randomised, double-blind phase II study
- PMID: 30616534
- PMCID: PMC6322336
- DOI: 10.1186/s12885-018-5224-6
Patients with metastatic renal cell carcinoma who benefit from axitinib dose titration: analysis from a randomised, double-blind phase II study
Abstract
Background: A prospective, randomised phase II study demonstrated clinical benefit of axitinib dose titration in a subset of treatment-naïve patients treated with axitinib for metastatic renal cell carcinoma. This analysis evaluated patient baseline characteristics that may impact overall survival (OS) with axitinib dose titration.
Methods: Following a 4-week lead-in period during which all patients received axitinib 5 mg twice-daily (bid); patients meeting the predefined randomisation criteria were randomly assigned to receive axitinib 5 mg bid plus either axitinib or placebo titration. In exploratory analyses, patients were grouped into those who achieved OS ≥24 versus < 24 months, and compared their baseline characteristics with Fisher's exact test or Cochran-Armitage trend exact test, with a 5% significance level. Potential predictive baseline characteristics associated with effect of axitinib dose titration on OS were investigated using a Cox proportional hazard model.
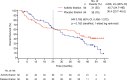
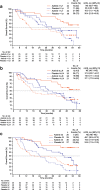
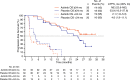
Results: Overall, 112 patients were randomised. Three of 56 patients receiving axitinib titration were censored; of the remaining 53, 33 (62%) achieved OS ≥24 months versus 20 (38%) with OS < 24 months. Patients with OS ≥24 vs. < 24 months, respectively, had significantly fewer metastatic sites (≤2 metastases: 52% vs. 10%; ≥3 metastases: 48% vs. 90%), fewer lymph node (45% vs. 75%) or liver (15% vs. 45%) metastases, higher haemoglobin level (i.e., ≥ lower limit of normal: 67% vs. 25%) at baseline, lower neutrophil (≤ upper limit of normal, 97% vs. 75%) and platelet (≤ upper limit of normal, 82% vs. 50%) levels at baseline and ≥ 1 year between histopathological diagnosis and treatment (64% vs. 15%). The primary reason for treatment discontinuation in both OS groups was disease progression. The frequency of toxicity-related discontinuation was comparable between the 2 groups, indicating that it was not a factor for a shorter OS. The multivariate analysis showed that ≥1 year from histopathological diagnosis to treatment and baseline haemoglobin level equal or greater than lower limit of normal were significant covariates associated with favourable OS in patients receiving axitinib titration.
Conclusions: The current analyses identified potentially predictive factors that could help selecting patients who may benefit from axitinib dose titration.
Trial registration: ClinicalTrials.gov identifier, NCT00835978. Registered prospectively, February 4, 2009.
Keywords: Axitinib; Benefit with dose titration; First-line; Metastatic renal cell carcinoma; Predictive factors; Survival benefit.
Conflict of interest statement
Authors’ information
None
Ethics approval and consent to participate
The study protocol and patient informed consent form were approved by the institutional review board or independent ethics committee at each centre. The institutional review boards or independent ethics committees included Eticka komise, Multicenticka eticka komise Fakultni nemocnice Olomouc, Eticka komise Fakultni nemocnice Na Bulovce, Eticka komise, and Masarykuv onkologicky usta y (Czech Republic); Ethikkommission des Fachbereichs Medizin der Johann Wolfgang (Germany); Yamagata University Hospital IRB, Kinki University Hospital IRB, Keio University Hospital IRB, Tokushima University Hospital IRB, Kyushu University Hospital IRB, Sapporo Medical University Hospital IRB, Hokkaido University Hospital IRB, Chiba Cancer Centre IRB, Hamamatsu University School of Medicine, University Hospital IRB, Kobe University Hospital IRB, Nagoya University Hospital IRB, Akita University Hospital IRB, Japanese Foundation For Cancer Research Cancer Institute Hospital IRB, National Cancer Centre IRB, and Nagasaki University Hospital IRB (Japan); Ethics Council at the Ministry of Healthcare and Social Development of Russian Federation, Local Ethics Committee at Russian Research Centre of Roentgenology and Radiology, Ethics Committee, Russian Oncological Research Centre n.a. Blokhin RAMS, Ethics Committee at Samara Regional Clinical Oncology Dispensary, Local ethical committee Institution of Russian Academy of Medical Sciences Medical Radiology Research Centre of RAMS, Ethics Committee Leningrad Regional Oncology Dispensary, Ethics Committee at the City Clinical Oncology Dispensary of Saint- Petersburg, and Ethics Committee at the Federal Service on Surveillance in Healthcare and Social Development (Russian Federation); CEIC AREA 5-Hospital Universitario La Paz Ethics Committee of Clinic Hospital General, Hospital Gregorio Marañon Comite Etico de Investigacion Clinica, and Hospital Universitario La Paz Ethics Committee of Clinic Investigation (Spain); Nebraska Methodist Hospital Institutional Review Board, Office for Human Research Studies Dana Farber Cancer Institute, Cleveland Clinic Institutional Review Board, University of Nebraska Medical Centre Institutional Review Board, The Johns Hopkins Medicine Institutional Review Board, Quorurn Review, Schulman Associates Institutional Review Board, Inc., IUPUI Institutional Review Board, Indiana University Institutional Review Board, Western Institutional Review Board, Washington University School of Medicine Human Research Protection Office (IRB), University of Texas M.D. Anderson Cancer Centre Surveillance Committee FWA-363, Oregon Health and Science University Research Integrity Office (ORIO) Institutional Review Board, and University of Cincinnati Institutional Review Board (USA). Written informed consent was obtained from each patient.
Consent for publication
Not applicable since the manuscript does not contain any individual patient data.
Competing interests
YT has received research funding from Pfizer, Ono, Takeda and Astellas, and honoraria from Pfizer, Novartis, Ono, Astellas and Bristol-Myers Squibb. HU has received research funding from Pfizer and Novartis, and honoraria from Pfizer, Bayer, Novartis, Ono and Bristol-Myers Squibb. MO has received research funding and honoraria from Pfizer, Novartis and Ono, and honoraria from Bayer. NS has received research funding from Pfizer, Ono and Astellas, and honoraria from Pfizer, Bayer, Novartis and GlaxoSmithKline. TH has received research funding and honoraria from Pfizer, Novartis and Bayer. YF, YK, and YU are employed by Pfizer R&D Japan and YU owns stock in Pfizer. AHB is employed by and owns stock in Pfizer. BIR has served as a consultant for and received research funding from Pfizer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Inlyta® (axitinib) US prescribing information. Pfizer Inc, New York, NY. Pfizer Inc. 2014. http://labeling.pfizer.com/ShowLabeling.aspx?id=759. Accessed 20 July 2017.
-
- Rini BI, Melichar B, Ueda T, Grunwald V, Fishman MN, Arranz JA, Bair AH, Pithavala YK, Andrews GI, Pavlov D, Kim S, Jonasch E. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncol. 2013;14(12):1233–1242. doi: 10.1016/S1470-2045(13)70464-9. - DOI - PMC - PubMed
-
- Rini BI, Tomita Y, Melichar B, Ueda T, Grunwald V, Fishman MN, Uemura H, Oya M, Bair AH, Andrews GI, Rosbrook B, Jonasch E. Overall survival analysis from a randomized phase II study of axitinib with or without dose titration in first-line metastatic renal cell carcinoma. Clin Genitourin Cancer. 2016;14(6):499–503. doi: 10.1016/j.clgc.2016.04.005. - DOI - PubMed
-
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

