HIV-1 molecular transmission clusters in nine European countries and Canada: association with demographic and clinical factors
- PMID: 30616632
- PMCID: PMC6323837
- DOI: 10.1186/s12916-018-1241-1
HIV-1 molecular transmission clusters in nine European countries and Canada: association with demographic and clinical factors
Abstract
Background: Knowledge of HIV-1 molecular transmission clusters (MTCs) is important, especially in large-scale datasets, for designing prevention programmes and public health intervention strategies. We used a large-scale HIV-1 sequence dataset from nine European HIV cohorts and one Canadian, to identify MTCs and investigate factors associated with the probability of belonging to MTCs.
Methods: To identify MTCs, we applied maximum likelihood inferences on partial pol sequences from 8955 HIV-positive individuals linked to demographic and clinical data. MTCs were defined using two different criteria: clusters with bootstrap support >75% (phylogenetic confidence criterion) and clusters consisting of sequences from a specific region at a proportion of >75% (geographic criterion) compared to the total number of sequences within the network. Multivariable logistic regression analysis was used to assess factors associated with MTC clustering.
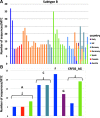
Results: Although 3700 (41%) sequences belonged to MTCs, proportions differed substantially by country and subtype, ranging from 7% among UK subtype C sequences to 63% among German subtype B sequences. The probability of belonging to an MTC was independently less likely for women than men (OR = 0.66; P < 0.001), older individuals (OR = 0.79 per 10-year increase in age; P < 0.001) and people of non-white ethnicity (OR = 0.44; P < 0.001 and OR = 0.70; P = 0.002 for black and 'other' versus white, respectively). It was also more likely among men who have sex with men (MSM) than other risk groups (OR = 0.62; P < 0.001 and OR = 0.69; P = 0.002 for people who inject drugs, and sex between men and women, respectively), subtype B (ORs 0.36-0.70 for A, C, CRF01 and CRF02 versus B; all P < 0.05), having a well-estimated date of seroconversion (OR = 1.44; P < 0.001), a later calendar year of sampling (ORs 2.01-2.61 for all post-2002 periods versus pre-2002; all P < 0.01), and being naïve to antiretroviral therapy at sampling (OR = 1.19; P = 0.010).
Conclusions: A high proportion (>40%) of individuals belonged to MTCs. Notably, the HIV epidemic dispersal appears to be driven by subtype B viruses spread within MSM networks. Expansion of regional epidemics seems mainly associated with recent MTCs, rather than the growth of older, established ones. This information is important for designing prevention and public health intervention strategies.
Keywords: Clusters; HIV; HIV epidemic; Molecular epidemiology; Phylogenies; Regional epidemics; Transmission networks.
Conflict of interest statement
Ethics approval and consent to participate
List of approval numbers/references from all participating cohorts:
Austrian participating centres: Ethikkommission der Medizinische Universitat Graz (21-431 ex09/10); Ethikkommission der Medizinischen Universitat Innsbruck (283/4.4); Ethikkommission des Landes Oberosterreich (C-3-10); Ethikkommission fur das Bundesland Salzburg (415-E/1159/4-2010); Ethikkommission der Medizinischen Universitat Wien (898/2010).
Canada registry: University of Calgary Medical Bioethics committee, Calgary Alberta Canada (REB15-0929_REN4 2).
French cohort: Comite de protection des Personnes ile de France II (Am7323-13-1157).
German HIV-1 Seroconverter Cohort: The ethical committee of the Charité University Medicine Berlin (EA2/105/05).
Greek cohort (AMACS – Athens Multicenter AIDS Cohort Study): The Hellenic Center for Diseases Control and Prevention (4090/2005); Athens University Medical School EC (319/2005) and the National Organization of Medicines (NIS-23-01-05/ 2006).
Italian cohort: Local ethical committees from all sites contributing to the cohort (10/08/69/07 AP; 348/L; 1932; 261/07/CE; 0132246/67GP; 115/2007; V2016; 44 CE/MA/CM; 63687; 98/07; RP/246/CE; 29/CE; 111/350; 857/D; 26547; 217/CEP; 51460; 5522/08-RN/RV; 1926/32/07; 42847; 125/2007/0/OSS; 24781; 3811/AO/16; 3039/CE; 2070211; 60/INT/CEI/11263; 006; 589/A1376/CE/2007; 38/2007; 1296/08; 35-2016; 1272; CE/38/14; 263/CELAZIO1; 3617; 7925/16; 32/2007; 7713/R; 20/16).
Netherlands registry: the Medical Ethical Committee of the Amsterdam Medical Centre, the Netherlands (07/182 and 09/040).
Norwegian cohort: Regionale Komiteer for Medisinsk og Helsefaglig Forskningstetikk (REK) (2016/892/REK; 1.2006.1811 - S-0885).
Spain cohort: Comité de Ética de la Investigación y de Bienestar Animal del Instituto de Salud Carlos III (ISCIII) (CEI PI 01_2012-v2).
UK registry: The West Midlands – South Birmingham National Research Ethics Service (04/G2707/155).
Enrolled study patients gave written informed consent. A full list of the ethics committees that provided approval can be found in the in Additional file 3.
Consent for publication
Not applicable.
Competing interests
AB received an ΙΚΥ Fellowship of Excellence for Postdoctoral Research in Greece – Siemens Program for this work. LM has received grants from ANRS, Fondation de la Recherche Médicale, and the European Union (EU) Framework Programme 7 (FP7) through the Medical Research Council–University College London (UCL), UK, outside of the submitted work. JG has received honoraria as an ad hoc member of the national HIV advisory boards of Merck, Gilead and ViiV, outside of the submitted work. KP received funding from EU FP7 while conducting this study, as well as honoraria from ViiV Healthcare and Merck, Sharp and Dohme, outside of the submitted work. GT received funding from the EU while conducting this study, as well as grants from the European Centre for Disease Prevention and Control, the EU, UCL, EU structural funds and National Funds and Gilead Sciences Europe, outside of the submitted work. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS data 2017: UNAIDS; 2017. http://www.unaids.org/en/resources/documents/2017/2017_data_book - PubMed
-
- Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–181. doi: 10.1001/jama.2016.5148. - DOI - PubMed
-
- Vermund SH, Fidler SJ, Ayles H, Beyers N, Hayes RJ, Team HS. Can combination prevention strategies reduce HIV transmission in generalized epidemic settings in Africa? The HPTN 071 (PopART) study plan in South Africa and Zambia. J Acq Imm Def. 2013;63:S221–S227. doi: 10.1097/QAI.0b013e318299c3f4. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

