Enteral versus intravenous approach for the sedation of critically ill patients: a randomized and controlled trial
- PMID: 30616675
- PMCID: PMC6323792
- DOI: 10.1186/s13054-018-2280-x
Enteral versus intravenous approach for the sedation of critically ill patients: a randomized and controlled trial
Abstract
Background: ICU patients must be kept conscious, calm, and cooperative even during the critical phases of illness. Enteral administration of sedative drugs might avoid over sedation, and would be as adequate as intravenous administration in patients who are awake, with fewer side effects and lower costs. This study compares two sedation strategies, for early achievement and maintenance of the target light sedation.
Methods: This was a multicenter, single-blind, randomized and controlled trial carried out in 12 Italian ICUs, involving patients with expected mechanical ventilation duration > 72 h at ICU admission and predicted mortality > 12% (Simplified Acute Physiology Score II > 32 points) during the first 24 h on ICU. Patients were randomly assigned to receive intravenous (midazolam, propofol) or enteral (hydroxyzine, lorazepam, and melatonin) sedation. The primary outcome was percentage of work shifts with the patient having an observed Richmond Agitation-Sedation Scale (RASS) = target RASS ±1. Secondary outcomes were feasibility, delirium-free and coma-free days, costs of drugs, length of ICU and hospital stay, and ICU, hospital, and one-year mortality.
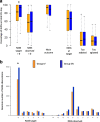
Results: There were 348 patients enrolled. There were no differences in the primary outcome: enteral 89.8% (74.1-100), intravenous 94.4% (78-100), p = 0.20. Enteral-treated patients had more protocol violations: n = 81 (46.6%) vs 7 (4.2%), p < 0.01; more self-extubations: n = 14 (8.1%) vs 4 (2.4%), p = 0.03; a lighter sedative target (RASS = 0): 93% (71-100) vs 83% (61-100), p < 0.01; and lower total drug costs: 2.39 (0.75-9.78) vs 4.15 (1.20-20.19) €/day with mechanical ventilation (p = 0.01).
Conclusions: Although enteral sedation of critically ill patients is cheaper and permits a lighter sedation target, it is not superior to intravenous sedation for reaching the RASS target.
Trial registration: ClinicalTrials.gov, NCT01360346 . Registered on 25 March 2011.
Keywords: Hydroxyzine; Hypnotics and sedatives; Melatonin; Nursing education research; Patient care planning.
Conflict of interest statement
Ethics approval and consent to participate
All participating centers obtained local ethics committee approvals to conduct the trial.
The coordinating center approval was issued by Comitato Etico of A.O. San Paolo - Polo Universitario, Milano: Prot.34/Reg.delibere 2011/CE on 23 February 2011.
The other ethics committees were:
Comitato Etico Interaziendale for the centers A.O.N. SS. Antonio e Biagio e Cesare Arrigo, Alessandria, and A.O. Ospedale Cardinal Massaia, Asti: Prot. 20848 on 16 September 2011
Comitato Etico of A.O. Ospedale Civile di Desio (MI): Prot. 17235 on 28 July 2011
Comitato Etico of A.O. Ospedale Civile di Legnano (MI): Prot. 15/12 on 18 May 2012
‘Comitato etico’ of N.O.C. Sant’Agostino Estense, Modena: Prot. 3083/CE of September 13, 2011.
Comitato Etico of A.O. San Gerardo, Monza (MB): Parere on 17 November 2011
Comitato Etico of A.O.U. San Luigi Gonzaga, Orbassano (TO): Prot. 18362 on 23 September 2011
Comitato Etico of I.R.C.C.S Ospedale Maggiore Policlinico, Milano: Parere on 13 December 2011
Comitato Etico of I.R.C.C.S. San Matteo, Pavia: Prot. 48199 on 3 March 2012
Comitato Etico of A.O. San Giovanni Bosco, Torino: Prot. 43718 on 3 August 2011
Written informed consent was mandatory for all able patients. When it could not be given, a written declaration of information received was collected from relatives, according to local ethics committee indications. As soon as patients’ neurological conditions improved, all enrolled patients were duly informed of the study and their written consent was obtained, both for the use of previously collected data, and for all prospective treatments and data collection. Patients or their next of kin could request withdrawal from the study at any time.
Consent for publication
Besides informed consent to participate, specific permission to use anonymized data for scientific purposes was collected from all patients.
Competing interests
All the authors, the Steering Committee members, and the SedaEN investigators declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. - DOI - PubMed
-
- Baron R, Binder A, Biniek R, Braune S, Buerkle H, Dall P, Demirakca S, Eckardt R, Eggers V, Eichler I, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) - short version. Ger Med Sci. 2015;13:Doc19. - PMC - PubMed
-
- Celis-Rodriguez E, Birchenall C, de la Cal MA, Castorena Arellano G, Hernandez A, Ceraso D, Diaz Cortes JC, Duenas Castell C, Jimenez EJ, Meza JC, et al. Clinical practice guidelines for evidence-based management of sedoanalgesia in critically ill adult patients. Med Int. 2013;37(8):519–574. - PubMed

