Cortical Dysmaturation in Congenital Heart Disease
- PMID: 30616953
- PMCID: PMC6397700
- DOI: 10.1016/j.tins.2018.12.003
Cortical Dysmaturation in Congenital Heart Disease
Abstract
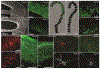
Congenital heart disease (CHD) is among the most common birth defects. Children with CHD frequently display long-term intellectual and behavioral disability. Emerging evidence indicates that cardiac anomalies lead to a reduction in cerebral oxygenation, which appears to profoundly impact on the maturation of cerebral regions responsible for higher-order cognitive functions. In this review we focus on the potential mechanisms by which dysregulation of cortical neuronal development during early life may lead to the significant cognitive impairments that commonly occur in children with CHD. Further understanding of the mechanisms underlying cortical dysmaturation due to CHD will be necessary to identify strategies for neonatal neuroprotection and for mitigating developmental delays in this patient population.
Keywords: congenital; cortex; heart; hypoxia; interneuron; perinatal.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

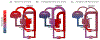
References
-
- Passingham RE and Smaers JB (2014) Is the prefrontal cortex especially enlarged in the human brain allometric relations and remapping factors. Brain. Behav. Evol. 84, 156–66 - PubMed
-
- Dubois J and Dehaene-Lambertz G (2015) Fetal and Postnatal Development of the Cortex: MRI and Genetics. Brain Mapp. An Encycl. Ref. 2, 11–19
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

