GPN does not release lysosomal Ca2+ but evokes Ca2+ release from the ER by increasing the cytosolic pH independently of cathepsin C
- PMID: 30617110
- PMCID: PMC6382017
- DOI: 10.1242/jcs.223883
GPN does not release lysosomal Ca2+ but evokes Ca2+ release from the ER by increasing the cytosolic pH independently of cathepsin C
Abstract
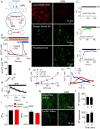
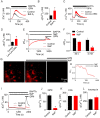
The dipeptide glycyl-l-phenylalanine 2-naphthylamide (GPN) is widely used to perturb lysosomes because its cleavage by the lysosomal enzyme cathepsin C is proposed to rupture lysosomal membranes. We show that GPN evokes a sustained increase in lysosomal pH (pHly), and transient increases in cytosolic pH (pHcyt) and Ca2+ concentration ([Ca2+]c). None of these effects require cathepsin C, nor are they accompanied by rupture of lysosomes, but they are mimicked by structurally unrelated weak bases. GPN-evoked increases in [Ca2+]c require Ca2+ within the endoplasmic reticulum (ER), but they are not mediated by ER Ca2+ channels amplifying Ca2+ release from lysosomes. GPN increases [Ca2+]c by increasing pHcyt, which then directly stimulates Ca2+ release from the ER. We conclude that physiologically relevant increases in pHcyt stimulate Ca2+ release from the ER in a manner that is independent of IP3 and ryanodine receptors, and that GPN does not selectively target lysosomes.
Keywords: Ca2+ signals; Cathepsin C; Cytosolic pH; Endoplasmic reticulum; GPN; Lysosome.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





Similar articles
-
The lysosomotrope GPN mobilises Ca2+ from acidic organelles.J Cell Sci. 2021 Mar 11;134(6):jcs256578. doi: 10.1242/jcs.256578. J Cell Sci. 2021. PMID: 33602742 Free PMC article.
-
Constitutive lysosome exocytosis releases ATP and engages P2Y receptors in human monocytes.J Cell Sci. 2012 Oct 1;125(Pt 19):4567-75. doi: 10.1242/jcs.107318. Epub 2012 Jul 5. J Cell Sci. 2012. PMID: 22767503
-
Does lysosomal rupture evoke Ca2+ release? A question of pores and stores.Cell Calcium. 2020 Mar;86:102139. doi: 10.1016/j.ceca.2019.102139. Epub 2019 Dec 9. Cell Calcium. 2020. PMID: 31881482 Review.
-
IP3 Receptors Preferentially Associate with ER-Lysosome Contact Sites and Selectively Deliver Ca2+ to Lysosomes.Cell Rep. 2018 Dec 11;25(11):3180-3193.e7. doi: 10.1016/j.celrep.2018.11.064. Cell Rep. 2018. PMID: 30540949 Free PMC article.
-
Ca(2+) channels on the move.Biochemistry. 2009 Dec 29;48(51):12062-80. doi: 10.1021/bi901739t. Biochemistry. 2009. PMID: 19928968 Free PMC article. Review.
Cited by
-
Nicotinic Acid Adenine Dinucleotide Phosphate Induces Intracellular Ca2+ Signalling and Stimulates Proliferation in Human Cardiac Mesenchymal Stromal Cells.Front Cell Dev Biol. 2022 Mar 15;10:874043. doi: 10.3389/fcell.2022.874043. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35392169 Free PMC article.
-
Ca2+-sensor ALG-2 engages ESCRTs to enhance lysosomal membrane resilience to osmotic stress.Proc Natl Acad Sci U S A. 2024 May 28;121(22):e2318412121. doi: 10.1073/pnas.2318412121. Epub 2024 May 23. Proc Natl Acad Sci U S A. 2024. PMID: 38781205 Free PMC article.
-
SOX9-induced Generation of Functional Astrocytes Supporting Neuronal Maturation in an All-human System.Stem Cell Rev Rep. 2021 Oct;17(5):1855-1873. doi: 10.1007/s12015-021-10179-x. Epub 2021 May 12. Stem Cell Rev Rep. 2021. PMID: 33982246 Free PMC article.
-
Contribution of NAADP to Glutamate-Evoked Changes in Ca2+ Homeostasis in Mouse Hippocampal Neurons.Front Cell Dev Biol. 2020 Jun 25;8:496. doi: 10.3389/fcell.2020.00496. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32676502 Free PMC article.
-
Initiation of acute pancreatitis in mice is independent of fusion between lysosomes and zymogen granules.Cell Mol Life Sci. 2024 May 6;81(1):207. doi: 10.1007/s00018-024-05247-7. Cell Mol Life Sci. 2024. PMID: 38709385 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

