Luspatercept improves hemoglobin levels and blood transfusion requirements in a study of patients with β-thalassemia
- PMID: 30617198
- PMCID: PMC6440118
- DOI: 10.1182/blood-2018-10-879247
Luspatercept improves hemoglobin levels and blood transfusion requirements in a study of patients with β-thalassemia
Abstract
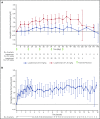
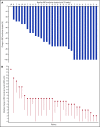
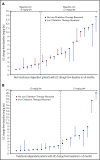
β-thalassemia is a hereditary disorder with limited approved treatment options; patients experience anemia and its complications, including iron overload. The study aim was to determine whether luspatercept could improve anemia and disease complications in patients with β-thalassemia. This open-label, nonrandomized, uncontrolled study consisted of a 24-week dose-finding and expansion stage (initial stage) and a 5-year extension stage, currently ongoing. Sixty-four patients were enrolled; 33 were non-transfusion dependent (mean hemoglobin, <10.0 g/dL; <4 red blood cell [RBC] units transfused per 8 weeks), and 31 were transfusion dependent (≥4 RBC units per 8 weeks). Patients received 0.2 to 1.25 mg/kg luspatercept subcutaneously every 21 days for ≥5 cycles (dose-finding stage) and 0.8 to 1.25 mg/kg (expansion cohort and 5-year extension). The primary end point was erythroid response, defined as hemoglobin increase of ≥1.5 g/dL from baseline for ≥14 consecutive days (without RBC transfusions) for non-transfusion-dependent patients or RBC transfusion burden reduction ≥20% over a 12-week period vs the 12 weeks before treatment for transfusion-dependent patients. Eighteen non-transfusion-dependent patients (58%) receiving higher dose levels of luspatercept (0.6-1.25 mg/kg) achieved mean hemoglobin increase ≥1.5 g/dL over ≥14 days vs baseline. Twenty-six (81%) transfusion-dependent patients achieved ≥20% reduction in RBC transfusion burden. The most common grade 1 to 2 adverse events were bone pain, headache, and myalgia. As of the cutoff, 33 patients remain on study. In this study, a high percentage of β-thalassemia patients receiving luspatercept had hemoglobin or transfusion burden improvements. These findings support a randomized clinical trial to assess efficacy and safety. This study was registered at www.clinicaltrials.gov as #NCT01749540 and #NCT02268409.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A. Piga has received research funding from Acceleron Pharma and Celgene Corporation; S.P. has received speaker’s honoraria from Novartis Oncology, has received research funding from Novartis Oncology and Acceleron Pharma, and has served on advisory boards at Bluebird Bio; E.V. has received research funding from Acceleron Pharma and Celgene Corporation; I.T. has received speaker’s honoraria from Novartis Oncology and has served on advisory boards at Bluebird Bio; C.B.-P. has received research funding from Acceleron Pharma and Celgene Corporation; X.Z., M.L.S., and K.M.A. are employed by and have equity ownership in Acceleron Pharma; and A.L. is employed by and has equity ownership in Celgene Corporation. The remaining authors declare no competing financial interests.
Figures

Comment in
-
A new medical therapy for anemia in thalassemia.Blood. 2019 Mar 21;133(12):1267-1268. doi: 10.1182/blood-2019-01-897587. Blood. 2019. PMID: 30898772 No abstract available.
References
-
- Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353(11):1135-1146. - PubMed
-
- Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood. 2011;118(13):3479-3488. - PubMed
-
- Origa R, Galanello R, Ganz T, et al. . Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583-588. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

