microRNA-22 promotes megakaryocyte differentiation through repression of its target, GFI1
- PMID: 30617215
- PMCID: PMC6325298
- DOI: 10.1182/bloodadvances.2018023804
microRNA-22 promotes megakaryocyte differentiation through repression of its target, GFI1
Abstract
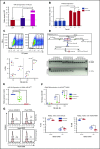
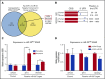
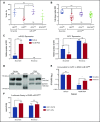
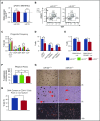
Precise control of microRNA expression contributes to development and the establishment of tissue identity, including in proper hematopoietic commitment and differentiation, whereas aberrant expression of various microRNAs has been implicated in malignant transformation. A small number of microRNAs are upregulated in megakaryocytes, among them is microRNA-22 (miR-22). Dysregulation of miR-22 leads to various hematologic malignancies and disorders, but its role in hematopoiesis is not yet well established. Here we show that upregulation of miR-22 is a critical step in megakaryocyte differentiation. Megakaryocytic differentiation in cell lines is promoted upon overexpression of miR-22, whereas differentiation is disrupted in CRISPR/Cas9-generated miR-22 knockout cell lines, confirming that miR-22 is an essential mediator of this process. RNA-sequencing reveals that miR-22 loss results in downregulation of megakaryocyte-associated genes. Mechanistically, we identify the repressive transcription factor, GFI1, as the direct target of miR-22, and upregulation of GFI1 in the absence of miR-22 inhibits megakaryocyte differentiation. Knocking down aberrant GFI1 expression restores megakaryocytic differentiation in miR-22 knockout cells. Furthermore, we have characterized hematopoiesis in miR-22 knockout animals and confirmed that megakaryocyte differentiation is similarly impaired in vivo and upon ex vivo megakaryocyte differentiation. Consistently, repression of Gfi1 is incomplete in the megakaryocyte lineage in miR-22 knockout mice and Gfi1 is aberrantly expressed upon forced megakaryocyte differentiation in explanted bone marrow from miR-22 knockout animals. This study identifies a positive role for miR-22 in hematopoiesis, specifically in promoting megakaryocyte differentiation through repression of GFI1, a target antagonistic to this process.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Similar articles
-
miR-125b modulates megakaryocyte maturation by targeting the cell-cycle inhibitor p19INK4D.Cell Death Dis. 2016 Oct 20;7(10):e2430. doi: 10.1038/cddis.2016.288. Cell Death Dis. 2016. PMID: 27763644 Free PMC article.
-
MicroRNA screen of human embryonic stem cell differentiation reveals miR-105 as an enhancer of megakaryopoiesis from adult CD34+ cells.Stem Cells. 2014 May;32(5):1337-46. doi: 10.1002/stem.1640. Stem Cells. 2014. PMID: 24446170 Free PMC article.
-
miR-34a contributes to megakaryocytic differentiation of K562 cells independently of p53.Blood. 2009 Sep 3;114(10):2181-92. doi: 10.1182/blood-2009-02-205062. Epub 2009 Jul 7. Blood. 2009. PMID: 19584398
-
microRNA regulation in megakaryocytopoiesis.Br J Haematol. 2011 Nov;155(3):298-307. doi: 10.1111/j.1365-2141.2011.08859.x. Epub 2011 Sep 13. Br J Haematol. 2011. PMID: 21910717 Review.
-
Transcription factor GATA-1 in megakaryocyte development.Stem Cells. 1998;16 Suppl 2:79-83. doi: 10.1002/stem.5530160710. Stem Cells. 1998. PMID: 11012179 Review.
Cited by
-
Post-Transcriptional Expression Control in Platelet Biogenesis and Function.Int J Mol Sci. 2020 Oct 15;21(20):7614. doi: 10.3390/ijms21207614. Int J Mol Sci. 2020. PMID: 33076269 Free PMC article. Review.
-
miR-486-5p and miR-22-3p Enable Megakaryocytic Differentiation of Hematopoietic Stem and Progenitor Cells without Thrombopoietin.Int J Mol Sci. 2022 May 11;23(10):5355. doi: 10.3390/ijms23105355. Int J Mol Sci. 2022. PMID: 35628168 Free PMC article.
-
Clinical Value of Serum miRNA in Patients with Acute Promyelocytic Leukemia.J Oncol. 2022 Apr 1;2022:7315879. doi: 10.1155/2022/7315879. eCollection 2022. J Oncol. 2022. PMID: 35401744 Free PMC article.
-
Clinical Significance of microRNAs in Hematologic Malignancies and Hematopoietic Stem Cell Transplantation.Cancers (Basel). 2023 May 8;15(9):2658. doi: 10.3390/cancers15092658. Cancers (Basel). 2023. PMID: 37174123 Free PMC article. Review.
-
Multi-omics and AI-driven advances in miRNA-mediated hair follicle regulation in cashmere goats.Front Vet Sci. 2025 Jul 9;12:1635202. doi: 10.3389/fvets.2025.1635202. eCollection 2025. Front Vet Sci. 2025. PMID: 40703926 Free PMC article. Review.
References
-
- Bluteau D, Lordier L, Di Stefano A, et al. . Regulation of megakaryocyte maturation and platelet formation. J Thromb Haemost. 2009;7(Suppl 1):227-234. - PubMed

