Increased Intracellular Cyclic di-AMP Levels Sensitize Streptococcus gallolyticus subsp. gallolyticus to Osmotic Stress and Reduce Biofilm Formation and Adherence on Intestinal Cells
- PMID: 30617242
- PMCID: PMC6398277
- DOI: 10.1128/JB.00597-18
Increased Intracellular Cyclic di-AMP Levels Sensitize Streptococcus gallolyticus subsp. gallolyticus to Osmotic Stress and Reduce Biofilm Formation and Adherence on Intestinal Cells
Abstract
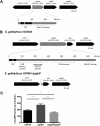
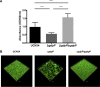
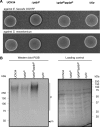
Cyclic di-AMP is a recently identified second messenger exploited by a number of Gram-positive bacteria to regulate important biological processes. Here, we studied the phenotypic alterations induced by the increased intracellular c-di-AMP levels in Streptococcus gallolyticus, an opportunistic pathogen responsible for septicemia and endocarditis in the elderly. We report that an S. gallolyticus c-di-AMP phosphodiesterase gdpP knockout mutant, which displays a 1.5-fold higher intracellular c-di-AMP levels than the parental strain UCN34, is more sensitive to osmotic stress and is morphologically smaller than the parental strain. Unexpectedly, we found that a higher level of c-di-AMP reduced biofilm formation of S. gallolyticus on abiotic surfaces and reduced adherence and cell aggregation on human intestinal cells. A genome-wide transcriptomic analysis indicated that c-di-AMP regulates many biological processes in S. gallolyticus, including the expression of various ABC transporters and disease-associated genes encoding bacteriocin and Pil3 pilus. Complementation of the gdpP in-frame deletion mutant with a plasmid carrying gdpP in trans from its native promoter restored bacterial morphology, tolerance to osmotic stress, biofilm formation, adherence to intestinal cells, bacteriocin production, and Pil3 pilus expression. Our results indicate that c-di-AMP is a pleiotropic signaling molecule in S. gallolyticus that may be important for S. gallolyticus pathogenesis.IMPORTANCEStreptococcus gallolyticus is an opportunistic pathogen responsible for septicemia and endocarditis in the elderly and is also strongly associated with colorectal cancer. S. gallolyticus can form biofilms, express specific pili to colonize the host tissues, and produce a specific bacteriocin allowing killing of commensal bacteria in the murine colon. Nevertheless, how the expression of these colonization factors is regulated remains largely unknown. Here, we show that c-di-AMP plays pleiotropic roles in S. gallolyticus, controlling the tolerance to osmotic stress, cell size, biofilm formation on abiotic surfaces, adherence and cell aggregation on human intestinal cells, expression of Pil3 pilus, and production of bacteriocin. This study indicates that c-di-AMP may constitute a key regulatory molecule for S. gallolyticus host colonization and pathogenesis.
Keywords: Streptococcus bovis; Streptococcus gallolyticus; biofilm; c-di-AMP; cell adherence.
Copyright © 2019 Teh et al.
Figures


Similar articles
-
Streptococcus gallolyticus Pil3 Pilus Is Required for Adhesion to Colonic Mucus and for Colonization of Mouse Distal Colon.J Infect Dis. 2015 Nov 15;212(10):1646-55. doi: 10.1093/infdis/jiv307. Epub 2015 May 26. J Infect Dis. 2015. PMID: 26014801
-
Functional analysis of c-di-AMP phosphodiesterase, GdpP, in Streptococcus suis serotype 2.Microbiol Res. 2014 Sep-Oct;169(9-10):749-58. doi: 10.1016/j.micres.2014.01.002. Epub 2014 Jan 26. Microbiol Res. 2014. PMID: 24680501
-
Heterogeneous expression of Pil3 pilus is critical for Streptococcus gallolyticus translocation across polarized colonic epithelial monolayers.Microbes Infect. 2020 Jan-Feb;22(1):55-59. doi: 10.1016/j.micinf.2019.12.001. Epub 2019 Dec 16. Microbes Infect. 2020. PMID: 31837399
-
The Road to Infection: Host-Microbe Interactions Defining the Pathogenicity of Streptococcus bovis/Streptococcus equinus Complex Members.Front Microbiol. 2018 Apr 10;9:603. doi: 10.3389/fmicb.2018.00603. eCollection 2018. Front Microbiol. 2018. PMID: 29692760 Free PMC article. Review.
-
[c-di-AMP regulates bacterial biofilm formation].Sheng Wu Gong Cheng Xue Bao. 2017 Sep 25;33(9):1369-1375. doi: 10.13345/j.cjb.170078. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 28956388 Review. Chinese.
Cited by
-
Increased Excess Intracellular Cyclic di-AMP Levels Impair Growth and Virulence of Bacillus anthracis.J Bacteriol. 2020 Apr 9;202(9):e00653-19. doi: 10.1128/JB.00653-19. Print 2020 Apr 9. J Bacteriol. 2020. PMID: 32071095 Free PMC article.
-
Ways to control harmful biofilms: prevention, inhibition, and eradication.Crit Rev Microbiol. 2021 Feb;47(1):57-78. doi: 10.1080/1040841X.2020.1842325. Epub 2020 Dec 28. Crit Rev Microbiol. 2021. PMID: 33356690 Free PMC article. Review.
-
Bacterial cell volume regulation and the importance of cyclic di-AMP.Microbiol Mol Biol Rev. 2024 Jun 27;88(2):e0018123. doi: 10.1128/mmbr.00181-23. Epub 2024 Jun 10. Microbiol Mol Biol Rev. 2024. PMID: 38856222 Free PMC article. Review.
-
c-di-AMP signaling is required for bile salt resistance, osmotolerance, and long-term host colonization by Clostridioides difficile.Sci Signal. 2022 Sep 6;15(750):eabn8171. doi: 10.1126/scisignal.abn8171. Epub 2022 Sep 6. Sci Signal. 2022. PMID: 36067333 Free PMC article.
-
Dysregulated biosynthesis and hydrolysis of cyclic-di-adenosine monophosphate impedes sporulation and butanol and acetone production in Clostridium beijerinckii NCIMB 8052.Front Bioeng Biotechnol. 2025 Feb 28;13:1547226. doi: 10.3389/fbioe.2025.1547226. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40092376 Free PMC article.
References
-
- Giannitsioti E, Chirouze C, Bouvet A, Beguinot I, Delahaye F, Mainardi JL, Celard M, Mihaila-Amrouche L, Moing VL, Hoen B. 2007. Characteristics and regional variations of group D streptococcal endocarditis in France. Clin Microbiol Infect 13:770–776. doi:10.1111/j.1469-0691.2007.01753.x. - DOI - PubMed
-
- Corredoira J, Alonso MP, Coira A, Casariego E, Arias C, Alonso D, Pita J, Rodriguez A, Lopez MJ, Varela J. 2008. Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur J Clin Microbiol Infect Dis 27:285–291. doi:10.1007/s10096-007-0441-y. - DOI - PubMed
-
- Selton-Suty C, Celard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B. 2012. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 54:1230. doi:10.1093/cid/cis199. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

