Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality
- PMID: 30617300
- PMCID: PMC6375784
- DOI: 10.1038/s41385-018-0115-3
Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality
Abstract
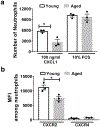
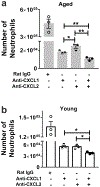
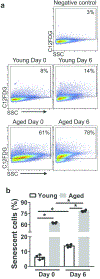
Neutrophils clear viruses, but excessive neutrophil responses induce tissue injury and worsen disease. Aging increases mortality to influenza infection; however, whether this is due to impaired viral clearance or a pathological host immune response is unknown. Here we show that aged mice have higher levels of lung neutrophils than younger mice after influenza viral infection. Depleting neutrophils after, but not before, infection substantially improves the survival of aged mice without altering viral clearance. Aged alveolar epithelial cells (AECs) have a higher frequency of senescence and secrete higher levels of the neutrophil-attracting chemokines CXCL1 and CXCL2 during influenza infection. These chemokines are required for age-enhanced neutrophil chemotaxis in vitro. Our work suggests that aging increases mortality from influenza in part because senescent AECs secrete more chemokines, leading to excessive neutrophil recruitment. Therapies that mitigate this pathological immune response in the elderly might improve outcomes of influenza and other respiratory infections.
Conflict of interest statement
Disclosure
The authors have declared that no conflict of interest exists.
Figures





References
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ et al. Mortality Associated With Influenza and Respiratory Syncytial Virus in the United States. JAMA: The Journal of the American Medical Association 2003; 289(2): 179–186. - PubMed
-
- Pebody RG, McLean E, Zhao H, Cleary P, Bracebridge S, Foster K et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Eurosurveillance 2010; 15(20): 19571. - PubMed
-
- Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in Hospitalizations for Pneumonia Among Persons Aged 65 Years or Older in the United States, 1988–2002. JAMA 2005; 294(21): 2712–2719. - PubMed
-
- Biggerstaff M, Kniss K, Jernigan DB, Brammer L, Bresee J, Garg S et al. Systematic Assessment of Multiple Routine and Near Real-Time Indicators to Classify the Severity of Influenza Seasons and Pandemics in the United States, 2003–2004 Through 2015–2016. American Journal of Epidemiology 2018; 187(5): 1040–1050. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

