Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models
- PMID: 30617325
- PMCID: PMC6327967
- DOI: 10.1038/s41591-018-0275-4
Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models
Abstract
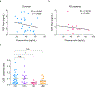
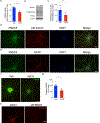
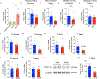
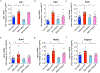
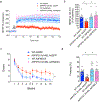
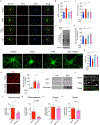
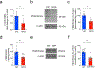
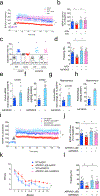
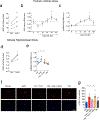
Defective brain hormonal signaling has been associated with Alzheimer's disease (AD), a disorder characterized by synapse and memory failure. Irisin is an exercise-induced myokine released on cleavage of the membrane-bound precursor protein fibronectin type III domain-containing protein 5 (FNDC5), also expressed in the hippocampus. Here we show that FNDC5/irisin levels are reduced in AD hippocampi and cerebrospinal fluid, and in experimental AD models. Knockdown of brain FNDC5/irisin impairs long-term potentiation and novel object recognition memory in mice. Conversely, boosting brain levels of FNDC5/irisin rescues synaptic plasticity and memory in AD mouse models. Peripheral overexpression of FNDC5/irisin rescues memory impairment, whereas blockade of either peripheral or brain FNDC5/irisin attenuates the neuroprotective actions of physical exercise on synaptic plasticity and memory in AD mice. By showing that FNDC5/irisin is an important mediator of the beneficial effects of exercise in AD models, our findings place FNDC5/irisin as a novel agent capable of opposing synapse failure and memory impairment in AD.
Figures

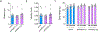
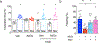




Comment in
-
An exercise-induced messenger boosts memory in Alzheimer's disease.Nat Med. 2019 Jan;25(1):20-21. doi: 10.1038/s41591-018-0311-4. Nat Med. 2019. PMID: 30617321 No abstract available.
References
-
- Prince M, et al. The global prevalence of dementia: a systematic review and meta-analysis. Alzheimer’s & Dementia 9, 63–75 e62 (2013). - PubMed
-
- Fernandez AM & Torres-Alemán I The many faces of insulin-like peptide signalling in the brain. Nature Reviews Neuroscience 13, 225–239 (2012). - PubMed
-
- Biessels GJ & Reagan LP Hippocampal insulin resistance and cognitive dysfunction. Nature Reviews Neuroscience 16, 660–671 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

