Cardiac Myosin-Binding Protein C-From Bench to Improved Diagnosis of Acute Myocardial Infarction
- PMID: 30617437
- PMCID: PMC6509074
- DOI: 10.1007/s10557-018-6845-3
Cardiac Myosin-Binding Protein C-From Bench to Improved Diagnosis of Acute Myocardial Infarction
Abstract
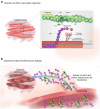
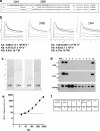
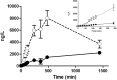
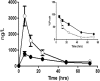
Chest pain is responsible for 6-10% of all presentations to acute healthcare providers. Triage is inherently difficult and heavily reliant on the quantification of cardiac Troponin (cTn), as a minority of patients with an ultimate diagnosis of acute myocardial infarction (AMI) present with clear diagnostic features such as ST-elevation on the electrocardiogram. Owing to slow release and disappearance of cTn, many patients require repeat blood testing or present with stable but elevated concentrations of the best available biomarker and are thus caught at the interplay of sensitivity and specificity.We identified cardiac myosin-binding protein C (cMyC) in coronary venous effluent and developed a high-sensitivity assay by producing an array of monoclonal antibodies and choosing an ideal pair based on affinity and epitope maps. Compared to high-sensitivity cardiac Troponin (hs-cTn), we demonstrated that cMyC appears earlier and rises faster following myocardial necrosis. In this review, we discuss discovery and structure of cMyC, as well as the migration from a comparably insensitive to a high-sensitivity assay facilitating first clinical studies. This assay was subsequently used to describe relative abundance of the protein, compare sensitivity to two high-sensitivity cTn assays and test diagnostic performance in over 1900 patients presenting with chest pain and suspected AMI. A standout feature was cMyC's ability to more effectively triage patients. This distinction is likely related to the documented greater abundance and more rapid release profile, which could significantly improve the early triage of patients with suspected AMI.
Keywords: AMI; Acute myocardial infarction; Biomarkers; Cardiac myosin-binding protein C; Cardiac troponin; Chest pain; Triage; cMyC.
Conflict of interest statement
Conflict of Interest
T.E.K. and B.A. have received research grants from the British Heart Foundation. T.E.K. has received speaker fees from Astra Zeneca. M.M. is named as an inventor on a patent held by King’s College London for the detection of cardiac myosin–binding protein C as a biomarker of myocardial injury.
Ethical Approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Figures
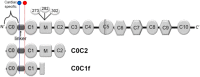

References
-
- Harris T, McDonald K. Is the case-mix of patients who self-present to ED similar to general practice and other acute-care facilities? Emerg Med J. 2014;31(12):970–974. - PubMed
-
- NHS Digital. Hospital Episode Statistics [Internet]. [cited 2018 Apr 30]. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

