Cellular Uptake of MCT1 Inhibitors AR-C155858 and AZD3965 and Their Effects on MCT-Mediated Transport of L-Lactate in Murine 4T1 Breast Tumor Cancer Cells
- PMID: 30617815
- PMCID: PMC6466617
- DOI: 10.1208/s12248-018-0279-5
Cellular Uptake of MCT1 Inhibitors AR-C155858 and AZD3965 and Their Effects on MCT-Mediated Transport of L-Lactate in Murine 4T1 Breast Tumor Cancer Cells
Abstract
AR-C155858 and AZD3965, pyrrole pyrimidine derivatives, represent potent monocarboxylate transporter 1 (MCT1) inhibitors, with potential immunomodulatory and chemotherapeutic properties. Currently, there is limited information on the inhibitory properties of this new class of MCT1 inhibitors. The purpose of this study was to characterize the concentration- and time-dependent inhibition of L-lactate transport and the membrane permeability properties of AR-C155858 and AZD3965 in the murine 4T1 breast tumor cells that express MCT1. Our results demonstrated time-dependent inhibition of L-lactate uptake by AR-C155858 and AZD3965 with maximal inhibition occurring after a 5-min pre-incubation period and prolonged inhibition. Following removal of AR-C155858 or AZD3965 from the incubation buffer, inhibition of L-lactate uptake was only fully reversed after 3 and 12 h, respectively, indicating that these inhibitors are slowly reversible. The uptake of AR-C155858 was concentration-dependent in 4T1 cells, whereas the uptake of AZD3965 exhibited no concentration dependence over the range of concentrations examined. The uptake kinetics of AR-C155858 was best fitted to a Michaelis-Menten equation with a diffusional clearance component, P (Km = 0.399 ± 0.067 μM, Vmax = 4.79 ± 0.58 pmol/mg/min, and P = 0.330 ± 0.088 μL/mg/min). AR-C155858 uptake, but not AZD3965 uptake, was significantly inhibited by alpha-cyano-4-hydroxycinnamic acid, a known nonspecific inhibitor of MCTs 1, 2, and 4. AR-C155858 demonstrated a trend toward higher uptake at lower pH, a characteristic of proton-dependent MCT1. These findings provide evidence that AR-C155858 and AZD3965 exert slowly reversible inhibition of MCT1-mediated L-lactate uptake in 4T1 cells, with AR-C155858 representing a potential substrate of MCT1.
Keywords: AR-C155858; AZD3965; L-lactate; MCT1 inhibitor; Monocarboxylate transporter 1.
Figures



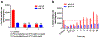

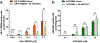

References
-
- Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Archiv. 2004;447(5):619–28. - PubMed
-
- Halestrap AP. The monocarboxylate transporter family–structure and functional characterization. IUBMB Life. 2012;64(1):1–9. - PubMed
-
- Halestrap AP, Wilson MC. The monocarboxylate transporter family–role and regulation. IUBMB Life. 2012;64(2):109–19. - PubMed
-
- Fishbein WN, Merezhinskaya N, Foellmer JW. Relative distribution of three major lactate transporters in frozen human tissues and their localization in unfixed skeletal muscle. Muscle Nerve. 2002;26(1):101–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

