The Combination of APRI and ALBI Facilitates Preoperative Risk Stratification for Patients Undergoing Liver Surgery After Neoadjuvant Chemotherapy
- PMID: 30617869
- PMCID: PMC6373283
- DOI: 10.1245/s10434-018-07125-6
The Combination of APRI and ALBI Facilitates Preoperative Risk Stratification for Patients Undergoing Liver Surgery After Neoadjuvant Chemotherapy
Abstract
Background: Neoadjuvant chemotherapy (NeoCTx) is performed for most patients with colorectal cancer liver metastases (CRCLM). However, chemotherapy-associated liver injury (CALI) has been associated with poor postoperative outcome. To date, however, no clinically applicable and noninvasive tool exists to assess CALI before liver resection.
Methods: Routine blood parameters were assessed in 339 patients before and after completion of NeoCTx and before surgery. The study assessed the prognostic potential of the aspartate aminotransferase (AST)-to-platelet ratio index (APRI), the albumin-bilirubin grade (ALBI), and their combinations. Furthermore, an independent multi-center validation cohort (n = 161) was included to confirm the findings concerning the prediction of postoperative outcome.
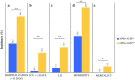
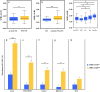
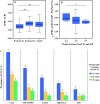
Results: Higher ALBI, APRI, and APRI + ALBI were found in patients with postoperative morbidity (P = 0.001, P = 0.064, P = 0.001, respectively), liver dysfunction (LD) (P = 0.009, P = 0.012, P < 0.001), or mortality (P = 0.037, P = 0.045, P = 0.016), and APRI + ALBI had the highest predictive potential for LD (area under the curve [AUC], 0.695). An increase in APRI + ALBI was observed during NeoCTx (P < 0.001). Patients with longer periods between NeoCTx and surgery showed a greater decrease in APRI + ALBI (P = 0.006) and a trend for decreased CALI at surgery. A cutoff for APRI + ALBI at - 2.46 before surgery was found to identify patients with CALI (P = 0.002) and patients at risk for a prolonged hospital stay (P = 0.001), intensive care (P < 0.001), morbidity (P < 0.001), LD (P < 0.001), and mortality (P = 0.021). Importantly, the study was able to confirm the predictive potential of APRI + ALBI for postoperative LD and mortality in a multicenter validation cohort.
Conclusion: Determination of APRI + ALBI before surgery enables identification of high-risk patients for liver resection. The combined score seems to dynamically reflect CALI. Thus, APRI + ALBI could be a clinically relevant tool for optimizing timing of surgery in CRCLM patients after NeoCTx.
Conflict of interest statement
The authors declare that they do not have anything to disclose regarding conflict of interest with respect to this manuscript. No funding or financial support was received for this work.
Figures


Comment in
-
Risikostratifizierung bei Patienten nach neoadjuvanter Chemotherapie.Zentralbl Chir. 2019 Apr;144(2):130-131. doi: 10.1055/a-0859-9736. Epub 2019 Apr 12. Zentralbl Chir. 2019. PMID: 30978758 German. No abstract available.
-
ASO Author Reflections: APRI + ALBI: A Novel Tool for Estimating Chemotherapy-Associated Liver Injury in Patients with Colorectal Cancer Liver Metastasis Undergoing Liver Resection.Ann Surg Oncol. 2019 Dec;26(Suppl 3):598-599. doi: 10.1245/s10434-019-07395-8. Epub 2019 Apr 29. Ann Surg Oncol. 2019. PMID: 31037436 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

