Clinical Trials on Diabetic Nephropathy: A Cross-Sectional Analysis
- PMID: 30617943
- PMCID: PMC6349284
- DOI: 10.1007/s13300-018-0551-9
Clinical Trials on Diabetic Nephropathy: A Cross-Sectional Analysis
Abstract
Introduction: Treatment options and decisions are often based on the results of clinical trials. We have evaluated the public availability of results from completed, registered phase III clinical trials on diabetic nephropathy and current treatment options.
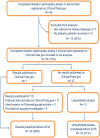
Methods: This was a cross-sectional analysis in which STrengthening the Reporting of OBservational studies in Epidemiology criteria were applied for design and analysis. In June 2017, 34 completed phase III clinical trials on diabetic nephropathy in the ClinicalTrials. gov registry were identified and matched to publications in the ClinicalTrials.gov registry and to those in the PubMed and Google Scholar databases. If no publication was identified, the principal investigator was contacted. The ratio of published and non-published studies was calculated. Various parameters, including study design, drugs, and comparators provided, were analyzed.
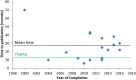
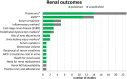
Results: Drugs/supplements belonged to 26 different categories of medications, with the main ones being angiotensin-converting enzyme inhibitors, angiotensin-II receptors blockers, and dipeptidyl-peptidase-4-inhibitors. Among the trials completed before 2016 (n = 32), 22 (69%) were published, and ten (31%) remained unpublished. Thus, data on 11 different interventions and more than 1000 patients remained undisclosed. Mean time to publication was 26.5 months, which is longer than the time constrictions imposed by the U.S. Food and Drug Administration Amendments Act. Most trials only showed weak effects on micro- and macroalbuminuria, with an absolute risk reduction of 1.0 and 0.3%, respectively, and the number needed to treat varied between 91 and 333, without any relevant effect on end-stage-renal disease by intensive glucose-lowering treatment. Comparison of the results, however, was difficult since study design, interventions, and the renal outcome parameters vary greatly between the studies.
Conclusion: Despite the financial and human resources involved and the relevance for therapeutic guidelines and clinical decisions, about one-third of phase III clinical trials on diabetic nephropathy remain unpublished. Interventions used in published trials showed a low efficacy on renal outcome.
Funding: Deutsche Forschungsgemeinschaft (DFG): SFB 1118.
Keywords: ACE inhibitors; Angiotensin-II receptors; ClinicalTrials.gov; Diabetes mellitus; Diabetic nephropathy; Dipeptidyl-peptidase-4-inhibitors; Phase III clinical trials.
Figures
Similar articles
-
Publication status of completed registered studies in paediatric appendicitis: a cross-sectional analysis.BMJ Open. 2018 Jul 16;8(7):e021684. doi: 10.1136/bmjopen-2018-021684. BMJ Open. 2018. PMID: 30012791 Free PMC article.
-
Pharmacologic management of diabetic nephropathy.Clin Ther. 2002 Nov;24(11):1741-56; discussion 1719. doi: 10.1016/s0149-2918(02)80076-5. Clin Ther. 2002. PMID: 12501871 Review.
-
Ten Years after the International Committee of Medical Journal Editors' Clinical Trial Registration Initiative, One Quarter of Phase 3 Pediatric Epilepsy Clinical Trials Still Remain Unpublished: A Cross Sectional Analysis.PLoS One. 2016 Jan 6;11(1):e0144973. doi: 10.1371/journal.pone.0144973. eCollection 2016. PLoS One. 2016. PMID: 26735955 Free PMC article.
-
Angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers for diabetic nephropathy: a retrospective comparison.J Renin Angiotensin Aldosterone Syst. 2009 Dec;10(4):195-200. doi: 10.1177/1470320309352352. J Renin Angiotensin Aldosterone Syst. 2009. PMID: 20026868
-
Clinical trial registration, reporting, publication and FDAAA compliance: a cross-sectional analysis and ranking of new drugs approved by the FDA in 2012.BMJ Open. 2015 Nov 12;5(11):e009758. doi: 10.1136/bmjopen-2015-009758. BMJ Open. 2015. PMID: 26563214 Free PMC article. Review.
Cited by
-
LncRNA SNHG17 knockdown promotes Parkin-dependent mitophagy and reduces apoptosis of podocytes through Mst1.Cell Cycle. 2020 Aug;19(16):1997-2006. doi: 10.1080/15384101.2020.1783481. Epub 2020 Jul 5. Cell Cycle. 2020. PMID: 32627655 Free PMC article.
-
Molecular mechanisms and therapeutic potential of natural flavonoids in diabetic nephropathy: Modulation of intracellular developmental signaling pathways.Curr Res Pharmacol Drug Discov. 2024 Jul 3;7:100194. doi: 10.1016/j.crphar.2024.100194. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 39071051 Free PMC article. Review.
-
Tetrazoles and Related Heterocycles as Promising Synthetic Antidiabetic Agents.Int J Mol Sci. 2023 Dec 6;24(24):17190. doi: 10.3390/ijms242417190. Int J Mol Sci. 2023. PMID: 38139019 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

