Red blood cells: the forgotten player in hemostasis and thrombosis
- PMID: 30618125
- PMCID: PMC6932746
- DOI: 10.1111/jth.14360
Red blood cells: the forgotten player in hemostasis and thrombosis
Abstract
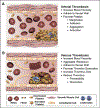
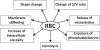
New evidence has stirred up a long-standing but undeservedly forgotten interest in the role of erythrocytes, or red blood cells (RBCs), in blood clotting and its disorders. This review summarizes the most recent research that describes the involvement of RBCs in hemostasis and thrombosis. There are both quantitative and qualitative changes in RBCs that affect bleeding and thrombosis, as well as interactions of RBCs with cellular and molecular components of the hemostatic system. The changes in RBCs that affect hemostasis and thrombosis include RBC counts or hematocrit (modulating blood rheology through viscosity) and qualitative changes, such as deformability, aggregation, expression of adhesive proteins and phosphatidylserine, release of extracellular microvesicles, and hemolysis. The pathogenic mechanisms implicated in thrombotic and hemorrhagic risk include variable adherence of RBCs to the vessel wall, which depends on the functional state of RBCs and/or endothelium, modulation of platelet reactivity and platelet margination, alterations of fibrin structure and reduced susceptibility to fibrinolysis, modulation of nitric oxide availability, and the levels of von Willebrand factor and factor VIII in blood related to the ABO blood group system. RBCs are involved in platelet-driven contraction of clots and thrombi that results in formation of a tightly packed array of polyhedral erythrocytes, or polyhedrocytes, which comprises a nearly impermeable barrier that is important for hemostasis and wound healing. The revisited notion of the importance of RBCs is largely based on clinical and experimental associations between RBCs and thrombosis or bleeding, implying that RBCs are a prospective therapeutic target in hemostatic and thrombotic disorders.
Keywords: blood clotting; erythrocytes; hemostasis; red blood cells; thrombosis.
© 2018 International Society on Thrombosis and Haemostasis.
Figures

References
-
- Duke WW. The relation of blood platelets to hemorrhagic disease. JAMA 1910; 60: 1185–92. - PubMed
-
- Tokish JM, Kocher MS, Hawkins RJ. Ergogenic aids: a review of basic science, performance, side effects, and status in sports. Am J Sports Med 2004; 32: 1543–53. - PubMed
-
- Barshtein G, Ben-Ami R, Yedgar S. Role of red blood cell flow behavior in hemodynamics and hemostasis. Expert Rev Cardiovasc Ther 2007; 5: 743–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

