Orexin Depolarizes Central Amygdala Neurons via Orexin Receptor 1, Phospholipase C and Sodium-Calcium Exchanger and Modulates Conditioned Fear
- PMID: 30618563
- PMCID: PMC6305451
- DOI: 10.3389/fnins.2018.00934
Orexin Depolarizes Central Amygdala Neurons via Orexin Receptor 1, Phospholipase C and Sodium-Calcium Exchanger and Modulates Conditioned Fear
Abstract
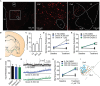
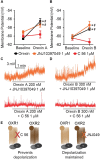
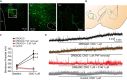
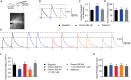
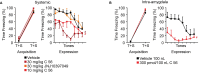
Orexins (OX), also known as hypocretins, are excitatory neuropeptides with well-described roles in regulation of wakefulness, arousal, energy homeostasis, and anxiety. An additional and recently recognized role of OX is modulation of fear responses. We studied the OX neurons of the perifornical hypothalamus (PeF) which send projections to the amygdala, a region critical in fear learning and fear expression. Within the amygdala, the highest density of OX-positive fibers was detected in the central nucleus (CeA). The specific mechanisms underlying OX neurotransmission within the CeA were explored utilizing rat brain slice electrophysiology, pharmacology, and chemogenetic stimulation. We show that OX induces postsynaptic depolarization of medial CeA neurons that is mediated by OX receptor 1 (OXR1) but not OX receptor 2 (OXR2). We further characterized the mechanism of CeA depolarization by OX as phospholipase C (PLC)- and sodium-calcium exchanger (NCX)- dependent. Selective chemogenetic stimulation of OX PeF fibers recapitulated OXR1 dependent depolarization of CeA neurons. We also observed that OXR1 activity modified presynaptic release of glutamate within the CeA. Finally, either systemic or intra-CeA perfusion of OXR1 antagonist reduced the expression of conditioned fear. Together, these data suggest the PeF-CeA orexinergic pathway can modulate conditioned fear through a signal transduction mechanism involving PLC and NCX activity and that selective OXR1 antagonism may be a putative treatment for fear-related disorders.
Keywords: central amygdala; chemogenetic; fear conditioning; orexin (hypocretin); orexin receptor 1 (OX1R).
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

