Retinal Neuroprotection From Optic Nerve Trauma by Deletion of Arginase 2
- PMID: 30618589
- PMCID: PMC6306467
- DOI: 10.3389/fnins.2018.00970
Retinal Neuroprotection From Optic Nerve Trauma by Deletion of Arginase 2
Abstract
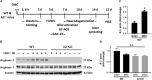
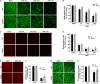
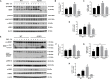
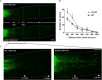
Our previous studies have implicated expression of the mitochondrial isoform of the arginase enzyme arginase 2 (A2) in neurovascular injury during ischemic retinopathies. The aim of this study was to characterize the specific involvement of A2 in retinal injury following optic nerve crush (ONC). To accomplish this, wild-type (WT) or A2 knockout (A2-/-) mice were subjected to ONC injury. The contralateral eye served as sham control. Quantitative RT-PCR and western blot were used to evaluate mRNA and protein expression. Retinal ganglion cell (RGC) survival was assessed in retinal whole mounts. Axonal sprouting was determined by anterograde transport of Cholera Toxin B (CTB). These analyses showed increased A2 expression following ONC. Numbers of NeuN-positive neurons as well as Brn3a- and RBPMS-positive RGC were decreased in the WT retinas at 14 days after ONC as compared to the sham controls. This ONC-induced neuronal loss was diminished in the A2-/- retinas. Similarly, axonal degeneration was ameliorated by A2 deletion whereas axon sprouting was enhanced. Significant retinal thinning was also seen in WT retinas at 21 days after ONC, and this was blocked in A2-/- mice. Cell death studies showed an increase in TUNEL positive cells in the RGC layer at 5 days after ONC in the WT retinas, and this was attenuated by A2 deletion. ONC increased glial cell activation in WT retinas, and this was significantly reduced by A2 deletion. Western blotting showed a marked increase in the neurotrophin, brain derived neurotrophic factor (BDNF) and its downstream signaling in A2-/- retinas vs. WT after ONC. This was associated with increases in the axonal regeneration marker GAP-43 in A2-/- retinas. Furthermore, A2-/- retinas showed decreased NLRP3 inflammasome activation and lower interleukin (IL-) 1β/IL-18 levels as compared to WT retinas subjected to ONC. Collectively, our results show that deletion of A2 limits ONC-induced neurodegeneration and glial activation, and enhances axonal sprouting by a mechanism involving increases in BDNF and decreases in retinal inflammation. These data demonstrate that A2 plays an important role in ONC-induced retinal damage. Blockade of A2 activity may offer a therapeutic strategy for preventing vision loss induced by traumatic retinal injury.
Keywords: arginase 2; brain derived neurotrophic factor; neuroprotection; optic nerve crush; retina; retinal ganglion cells.
Figures



References
-
- Fournier A. E., Beer J., Arregui C. O., Essagian C., Aguayo A. J., Mckerracher L. (1997). Brain-derived neurotrophic factor modulates GAP-43 but not T alpha1 expression in injured retinal ganglion cells of adult rats. J. Neurosci. Res. 47 561–572. 10.1002/(SICI)1097-4547(19970315)47:6<561::AID-JNR1>3.0.CO;2-B - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

