Direct Bypass Surgery Vs. Combined Bypass Surgery for Hemorrhagic Moyamoya Disease: A Comparison of Angiographic Outcomes
- PMID: 30619072
- PMCID: PMC6306562
- DOI: 10.3389/fneur.2018.01121
Direct Bypass Surgery Vs. Combined Bypass Surgery for Hemorrhagic Moyamoya Disease: A Comparison of Angiographic Outcomes
Abstract
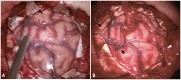
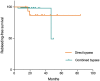
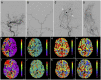
Objective: Extracranial-intracranial bypass is currently recognized as the optimal treatment for hemorrhagic-type moyamoya disease (MMD) which reduces incidence of rebleeding. Recent studies have reported the advantage of combined bypass over direct bypass for the general MMD patients. However, the effect of direct bypass and combined bypass surgery specifically for hemorrhagic-type MMD had not been investigated yet. Methods: Hemorrhagic-type MMD patients who underwent direct and combined bypass surgery with complete clinical and radiological documentation from a multicenter cohort between 2009 and 2017 were retrospectively included. Surgical methods included superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis (direct bypass), combined STA-MCA bypass with encephalodurosynangiosis (EDS), and combined STA-MCA bypass with encephaloduroarteriosynangiosis (EDAS). Matsushima standard on follow-up catheter angiography was used to assess surgical outcome. Modified Rankin Scale, incidence of rebleeding and ischemia during follow-up were recorded. Rebleeding-free survival rates between direct and combined bypass were compared by Kaplan-Meier analysis. Results: Sixty eight hemorrhagic-onset MMD patients were included in this study, among which 71 hemispheres were treated with surgery (direct bypass: 17; bypass+EDS: 24; bypass+EDAS: 30). Forty six (64.8%) hemispheres had satisfactory revascularization (Matsushima level 2-3) and 26 (36.6%) had poor neoangiogenesis. Matsushima level was not significantly different between surgical groups (P = 0.258). Good neoangiogenesis from dural grafts was achieved in 26 (36.6%) hemispheres, and good neoangiogenesis from STA grafts was only seen in 4 (out of 30, 12.5%) hemispheres. Multivariate analysis showed bypass patency [P < 0.001, OR (95%CI): 13.41 (3.28-54.80)] and dural neoangiogenesis [P < 0.001, OR (95%CI): 13.18 (3.26-53.36)] both independently contributed to good angiographic outcome. During follow-up, incidences of rebleeding or ischemic events, and re-bleeding free survival rate were not significantly different between surgical groups (P = 0.433, P = 0.559, and P = 0.997). However, patients who underwent combined bypass surgery had significantly lower mRS at follow-up comparing to patients who underwent direct bypass (P = 0.006). Conclusion: Combined bypass surgery and direct bypass surgery offered similar revascularization for hemorrhagic MMD. Bypass patency and dural angiogenesis both contributed to revascularization independently. The potential of indirect bypass to grow new vessels in hemorrhagic-MMD patients was generally limited, but dural leaflets offered better neoangiogenesis than STA grafts and was therefore recommended for surgical revascularization of hemorrhagic MMD.
Keywords: angiographic outcome; combined bypass; direct bypass; hemorrhagic-type; moyamoya disease; surgical outcome; surgical revascularization.
Figures




References
LinkOut - more resources
Full Text Sources

