Gene Editing of HIV-1 Co-receptors to Prevent and/or Cure Virus Infection
- PMID: 30619107
- PMCID: PMC6304358
- DOI: 10.3389/fmicb.2018.02940
Gene Editing of HIV-1 Co-receptors to Prevent and/or Cure Virus Infection
Abstract
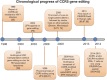
Antiretroviral therapy has prolonged the lives of people living with human immunodeficiency virus type 1 (HIV-1), transforming the disease into one that can be controlled with lifelong therapy. The search for an HIV-1 vaccine has plagued researchers for more than three decades with little to no success from clinical trials. Due to these failures, scientists have turned to alternative methods to develop next generation therapeutics that could allow patients to live with HIV-1 without the need for daily medication. One method that has been proposed has involved the use of a number of powerful gene editing tools; Zinc Finger Nucleases (ZFN), Transcription Activator-like effector nucleases (TALENs), and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 to edit the co-receptors (CCR5 or CXCR4) required for HIV-1 to infect susceptible target cells efficiently. Initial safety studies in patients have shown that editing the CCR5 locus is safe. More in depth in vitro studies have shown that editing the CCR5 locus was able to inhibit infection from CCR5-utilizing virus, but CXCR4-utilizing virus was still able to infect cells. Additional research efforts were then aimed at editing the CXCR4 locus, but this came with other safety concerns. However, in vitro studies have since confirmed that CXCR4 can be edited without killing cells and can confer resistance to CXCR4-utilizing HIV-1. Utilizing these powerful new gene editing technologies in concert could confer cellular resistance to HIV-1. While the CD4, CCR5, CXCR4 axis for cell-free infection has been the most studied, there are a plethora of reports suggesting that the cell-to-cell transmission of HIV-1 is significantly more efficient. These reports also indicated that while broadly neutralizing antibodies are well suited with respect to blocking cell-free infection, cell-to-cell transmission remains refractile to this approach. In addition to stopping cell-free infection, gene editing of the HIV-1 co-receptors could block cell-to-cell transmission. This review aims to summarize what has been shown with regard to editing the co-receptors needed for HIV-1 entry and how they could impact the future of HIV-1 therapeutic and prevention strategies.
Keywords: CCR5; CD4; CRISPR/Cas9; CXCR4; HIV-1.
Figures


References
-
- Abrahams M. R., Anderson J. A., Giorgi E. E., Seoighe C., Mlisana K., Ping L. H., et al. (2009). Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J. Virol. 83 3556–3567. 10.1128/JVI.02132-08 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

