T Lymphocytes Contribute to the Control of Baseline Neural Precursor Cell Proliferation but Not the Exercise-Induced Up-Regulation of Adult Hippocampal Neurogenesis
- PMID: 30619254
- PMCID: PMC6297802
- DOI: 10.3389/fimmu.2018.02856
T Lymphocytes Contribute to the Control of Baseline Neural Precursor Cell Proliferation but Not the Exercise-Induced Up-Regulation of Adult Hippocampal Neurogenesis
Abstract
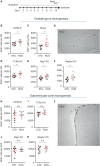
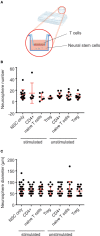
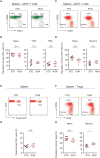
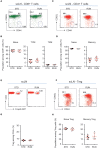
Cross-talk between the peripheral immune system and the central nervous system is important for physiological brain health. T cells are required to maintain normal baseline levels of neural precursor proliferation in the hippocampus of adult mice. We show here that neither T cells, B cells, natural killer cells nor natural killer T cells are required for the increase in hippocampal precursor proliferation that occurs in response to physical exercise. In addition, we demonstrate that a subpopulation of T cells, regulatory T cells, is not involved in maintaining baseline levels of neural precursor proliferation. Even when applied at supraphysiological numbers, populations of both naive and stimulated lymphocytes had no effect on hippocampal precursor proliferation in vitro. In addition, physical activity had no effect on peripheral immune cells in terms of distribution in the bone marrow, lymph nodes or spleen, activation state or chemokine receptor (CXCR4 and CCR9) expression. Together these results suggest that lymphocytes are not involved in translating the peripheral effects of exercise to the neurogenic niche in the hippocampus and further support the idea that the exercise-induced regulation of adult neurogenesis is mechanistically distinct from its baseline control.
Keywords: T cell; adult neurogenesis; dentate gyrus; hippocampus; neural precursor cell; physical activity; regulatory T cell.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

