Novel PLCG2 Mutation in a Patient With APLAID and Cutis Laxa
- PMID: 30619256
- PMCID: PMC6302768
- DOI: 10.3389/fimmu.2018.02863
Novel PLCG2 Mutation in a Patient With APLAID and Cutis Laxa
Abstract
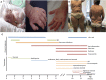
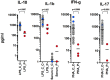
Background: The auto-inflammation and phospholipase Cγ2 (PLCγ2)-associated antibody deficiency and immune dysregulation (APLAID) syndrome is a rare primary immunodeficiency caused by a gain-of-function mutation S707Y in the PLCG2 gene previously described in two patients from one family. The APLAID patients presented with early-onset blistering skin lesions, posterior uveitis, inflammatory bowel disease (IBD) and recurrent sinopulmonary infections caused by a humoral defect, but lacked circulating autoantibodies and had no cold-induced urticaria, contrary to the patients with the related PLAID syndrome. Case: We describe a new APLAID patient who presented with vesiculopustular rash in the 1st weeks of life, followed by IBD, posterior uveitis, recurrent chest infections, interstitial pneumonitis, and also had sensorineural deafness and cutis laxa. Her disease has been refractory to most treatments, including IL1 blockers and a trial with ruxolitinib has been attempted. Results: In this patient, we found a unique de novo heterozygous missense L848P mutation in the PLCG2 gene, predicted to affect the PLCγ2 structure. Similarly to S707Y, the L848P mutation led to the increased basal and EGF-stimulated PLCγ2 activity in vitro. Whole blood assays showed reduced production of IFN-γ and IL-17 in response to polyclonal T-cell stimulation and reduced production of IL-10 and IL-1β after LPS stimulation. Reduced IL-1β levels and the lack of clinical response to treatment with IL-1 blockers argue against NLRP3 inflammasome hyperactivation being the main mechanism mediating the APLAID pathogenesis. Conclusion: Our findings indicate that L848P is novel a gain-of-function mutation that leads to PLCγ2 activation and suggest cutis laxa as a possible clinical manifestations of the APLAID syndrome.
Keywords: APLAID; IL-10; IL-1b; PLCγ2; auto-inflammatory syndromes; cutis laxa; sensorineural deafness.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

