Ovarian Cancer-Intrinsic Fatty Acid Synthase Prevents Anti-tumor Immunity by Disrupting Tumor-Infiltrating Dendritic Cells
- PMID: 30619288
- PMCID: PMC6302125
- DOI: 10.3389/fimmu.2018.02927
Ovarian Cancer-Intrinsic Fatty Acid Synthase Prevents Anti-tumor Immunity by Disrupting Tumor-Infiltrating Dendritic Cells
Abstract
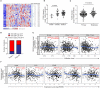
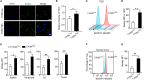
Fatty acid synthase (FASN), the key metabolic enzyme of de novo lipogenesis, provides proliferative and metastatic capacity directly to cancer cells have been described. However, the impact of aberrant activation of this lipogenic enzyme on host anti-tumor immune milieu remains unknown. In this study, we depicted that elevated FASN expression presented in ovarian cancer with more advanced clinical phenotype and correlated with the immunosuppressive status, which characterized by the lower number and dysfunction of infiltrating T cells. Notably, in a mouse model, we showed that tumor cell-intrinsic FASN drove ovarian cancer (OvCa) progression by blunting anti-tumor immunity. Dendritic cells (DCs) are required to initiate and sustain T cell-dependent anti-tumor immunity. Here, our data showed that constitutive activation of FASN in ovarian cancer cell lead to abnormal lipid accumulation and subsequent inhibition of tumor-infiltrating DCs (TIDCs) capacity to support anti-tumor T cells. Mechanistically, FASN activation in ovarian cancer cell-induced the resulting increase of lipids present at high concentrations in the tumor microenvironment. Dendritic cells educated by FASNhigh OvCa ascites are defective in their ability to present antigens and prime T cells. Accordingly, inhibiting FASN by FASN inhibitor can partly restore the immunostimulatory activity of TIDCs and extended tumor control by evoking protective anti-tumor immune responses. Therefore, our data provide a mechanism by which ovarian cancer-intrinsic FASN oncogenic pathway induce the impaired anti-tumor immune response through lipid accumulation in TIDCs and subsequently T-cells exclusion and dysfunction. These results could further indicate that targeting the FASN oncogenic pathway concomitantly enhance anti-tumor immunity, thus offering a unique approach to ovarian cancer immunotherapy.
Keywords: FASN; immune response; immunity; ovarian cancer; tumor-infiltrating dendritic cells.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

