Overcoming Target Driven Fratricide for T Cell Therapy
- PMID: 30619300
- PMCID: PMC6299907
- DOI: 10.3389/fimmu.2018.02940
Overcoming Target Driven Fratricide for T Cell Therapy
Abstract
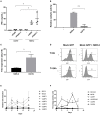
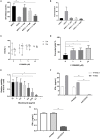
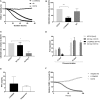
Chimeric Antigen Receptor (CAR) T cells expressing the fusion of the NKG2D protein with CD3ζ (NKG2D-CAR T Cells) acquire a specificity for stress-induced ligands expressed on hematological and solid cancers. However, these stress ligands are also transiently expressed by activated T cells implying that NKG2D-based T cells may undergo self-killing (fratricide) during cell manufacturing or during the freeze thaw cycle prior to infusion in patients. To avoid target-driven fratricide and enable the production of NKG2D-CAR T cells for clinical application, two distinct approaches were investigated. The first focused upon the inclusion of a Phosphoinositol-3-Kinase inhibitor (LY294002) into the production process. A second strategy involved the inclusion of antibody blockade of NKG2D itself. Both processes impacted T cell fratricide, albeit at different levels with the antibody process being the most effective in terms of cell yield. While both approaches generated comparable NKG2D-CAR T cells, there were subtle differences, for example in differentiation status, that were fine-tuned through the phasing of the inhibitor and antibody during culture in order to generate a highly potent NKG2D-CAR T cell product. By means of targeted inhibition of NKG2D expression or generic inhibition of enzyme function, target-driven CAR T fratricide can be overcome. These strategies have been incorporated into on-going clinical trials to enable a highly efficient and reproducible manufacturing process for NKG2D-CAR T cells.
Keywords: CD314; NKG2D; NKG2D blocking antibody; PI3K inhibitor; T cells; chimeric antigen receptor; fratricide.
Figures

References
-
- Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. . Durable molecular remissions in chronic lymphocytic leukemia treated with cd19-specific chimeric antigen receptor–modified t cells after failure of ibrutinib. J Clin Oncol. (2017) 35:3010–20. 10.1200/JCO.2017.72.8519 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

