The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure
- PMID: 30619308
- PMCID: PMC6302023
- DOI: 10.3389/fimmu.2018.02948
The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure
Abstract
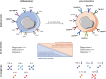
Acute and acute-on-chronic liver failure (ALF and ACLF), though distinct clinical entities, are considered syndromes of innate immune dysfunction. Patients with ALF and ACLF display evidence of a pro-inflammatory state with local liver inflammation, features of systemic inflammatory response syndrome (SIRS) and vascular endothelial dysfunction that drive progression to multi-organ failure. In an apparent paradox, these patients are concurrently immunosuppressed, exhibiting acquired immune defects that render them highly susceptible to infections. This paradigm of tissue injury succeeded by immunosuppression is seen in other inflammatory conditions such as sepsis, which share poor outcomes and infective complications that account for high morbidity and mortality. Monocyte and macrophage dysfunction are central to disease progression of ALF and ACLF. Activation of liver-resident macrophages (Kupffer cells) by pathogen and damage associated molecular patterns leads to the recruitment of innate effector cells to the injured liver. Early monocyte infiltration may contribute to local tissue destruction during the propagation phase and results in secretion of pro-inflammatory cytokines that drive SIRS. In the hepatic microenvironment, recruited monocytes mature into macrophages following local reprogramming so as to promote resolution responses in a drive to maintain tissue integrity. Intra-hepatic events may affect circulating monocytes through spill over of soluble mediators and exposure to apoptotic cell debris during passage through the liver. Hence, peripheral monocytes show numerous acquired defects in acute liver failure syndromes that impair their anti-microbial programmes and contribute to enhanced susceptibility to sepsis. This review will highlight the cellular and molecular mechanisms by which monocytes and macrophages contribute to the pathophysiology of ALF and ACLF, considering both hepatic inflammation and systemic immunosuppression. We identify areas for further research and potential targets for immune-based therapies to treat these devastating conditions.
Keywords: acute liver failure; acute-on-chronic liver failure; damage-associated molecular patterns; immunosuppression; liver inflammation; macrophages; monocytes; pathogen-associated molecular patterns.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

