Systemic and Tissue Inflammation in Juvenile Dermatomyositis: From Pathogenesis to the Quest for Monitoring Tools
- PMID: 30619311
- PMCID: PMC6305419
- DOI: 10.3389/fimmu.2018.02951
Systemic and Tissue Inflammation in Juvenile Dermatomyositis: From Pathogenesis to the Quest for Monitoring Tools
Abstract
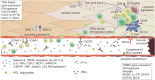
Juvenile Dermatomyositis (JDM) is a systemic immune-mediated disease of childhood, characterized by muscle weakness, and a typical skin rash. Other organ systems and tissues such as the lungs, heart, and intestines can be involved, but may be under-evaluated. The inflammatory process in JDM is characterized by an interferon signature and infiltration of immune cells such as T cells and plasmacytoid dendritic cells into the affected tissues. Vasculopathy due to loss and dysfunction of endothelial cells as a result of the inflammation is thought to underlie the symptoms in most organs and tissues. JDM is a heterogeneous disease, and several disease phenotypes, each with a varying combination of affected tissues and organs, are linked to the presence of myositis autoantibodies. These autoantibodies have therefore been extensively studied as biomarkers for the disease phenotype and its associated prognosis. Next to identifying the JDM phenotype, monitoring of disease activity and disease-inflicted damage not only in muscle and skin, but also in other organs and tissues, is an important part of clinical follow-up, as these are key determinants for the long-term outcomes of patients. Various monitoring tools are currently available, among which clinical assessment, histopathological investigation of muscle and skin biopsies, and laboratory testing of blood for specific biomarkers. These investigations also give novel insights into the underlying immunological processes that drive inflammation in JDM and suggest a strong link between the interferon signature and vasculopathy. New tools are being developed in the quest for minimally invasive, but sensitive and specific diagnostic methods that correlate well with clinical symptoms or reflect local, low-grade inflammation. In this review we will discuss the types of (extra)muscular tissue inflammation in JDM and their relation to vasculopathic changes, critically assess the available diagnostic methods including myositis autoantibodies and newly identified biomarkers, and reflect on the immunopathogenic implications of identified markers.
Keywords: autoantibodies; biomarkers; disease monitoring; interferon signature; juvenile dermatomyositis; personalized medicine; tissue inflammation; vasculopathy.
Figures
References
-
- Rothwell S, Cooper RG, Lundberg IE, Miller FW, Gregersen PK, Bowes J, et al. . Dense genotyping of immune-related loci in idiopathic inflammatory myopathies confirms HLA alleles as the strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Ann Rheum Dis. (2016) 75:1558–66. 10.1136/annrheumdis-2015-208119 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

