Inhibition of Inflammatory Changes in Human Myometrial Cells by Cell Penetrating Peptide and Small Molecule Inhibitors of NFκB
- PMID: 30619324
- PMCID: PMC6307458
- DOI: 10.3389/fimmu.2018.02966
Inhibition of Inflammatory Changes in Human Myometrial Cells by Cell Penetrating Peptide and Small Molecule Inhibitors of NFκB
Abstract
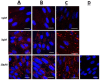
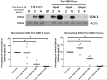
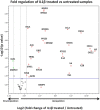
Complications arising from Preterm Birth are the leading causes of neonatal death globally. Current therapeutic strategies to prevent Preterm Birth are yet to demonstrate success in terms of reducing this neonatal disease burden. Upregulation of intracellular inflammatory pathways in uterine cells, including those involving nuclear factor kappa-B (NFκB), have been causally linked to both human term and preterm labor, but the barrier presented by the cell membrane presents an obstacle to interventions aimed at dampening these inflammatory responses. Cell penetrating peptides (CPPs) are novel vectors that can traverse cell membranes without the need for recognition by cell surface receptors and offer the ability to deliver therapeutic cargo internal to cell membranes. Using a human uterine cell culture inflammatory model, this study aimed to test the effectiveness of CPP-cargo delivery to inhibit inflammatory responses, comparing this effect with a small molecule inhibitor (Sc514) that has a similar intracellular target of action within the NFκB pathway (the IKK complex). The CPP Penetratin, conjugated to rhodamine, was able to enter uterine cells within a 60 min timeframe as assessed by live confocal microscopy, this phenomena was not observed with the use of a rhodamine-conjugated inert control peptide (GC(GS)4). Penetratin CPP conjugated to an IKK-inhibitory peptide (Pen-NBD) demonstrated ability to inhibit both the IL1β-induced expression of the inflammatory protein COX2 and dampen the expression of a bespoke array of inflammatory genes. Truncation of the CPP vector rendered the CPP-cargo conjugate much less effective, demonstrating the importance of careful vector selection. The small molecule inhibitor Sc514 also demonstrated ability to inhibit COX2 protein responses and a broad down-regulatory effect on uterine cell inflammatory gene expression. These results support the further exploration of either CPP-based or small molecular treatment strategies to dampen gestational cell inflammatory responses in the context of preterm birth. The work underlines both the importance of careful selection of CPP vector-cargo combinations and basic testing over a broad time and concentration range to ensure effective responses. Further work should demonstrate the effectiveness of CPP-linked cargos to dampen alternative pathways of inflammation linked to Preterm Birth such as MAP Kinase or AP1.
Keywords: cell penetrating peptide; myometrial cell; nuclear factor kappa B; preterm birth; tocolytic.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

