HMGN1 and R848 Synergistically Activate Dendritic Cells Using Multiple Signaling Pathways
- PMID: 30619338
- PMCID: PMC6305469
- DOI: 10.3389/fimmu.2018.02982
HMGN1 and R848 Synergistically Activate Dendritic Cells Using Multiple Signaling Pathways
Abstract
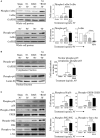
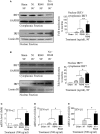
High mobility group nucleosome-binding protein 1 (HMGN1 or N1) is a Th1-polarizing alarmin, but alone is insufficient to induce antitumor immunity. We previously showed that combination of N1 and R848, a synthetic TLR7/8 agonist, synergistically activates dendritic cells (DCs) and induces therapeutic antitumor immunity, however, it remained unclear how N1 and R848 synergistically activate DCs. Here, we show that co-stimulation with N1 and R848 of human monocyte-derived DCs (MoDCs) markedly upregulated DC's surface expression of CD80, CD83, CD86, and HLA-DR, as well as synergistic production of pro-inflammatory cytokines including IL-12p70, IL-1β, and TNF-α. This combination also synergistically activated NF-κB and multiple MAPKs that are involved in DC maturation. Moreover, N1 and R848 synergistically increased nuclear translocation of interferon (IFN) regulatory transcription factors (e.g., IRF3 and IRF7) and promoted the expression of type 1 IFNs such as IFN-α2, IFN-α4, and IFN-β1. Similar signaling pathways were also induced in mouse bone marrow-derived DCs (BMDCs). RNA-seq analysis in human MoDCs revealed that N1 plus R848 synergistically upregulated the expression of genes predominantly involved in DC maturation pathway, particularly genes critical for the polarization of Th1 immune responses (e.g., IL12A, IL12B, and IFNB1, etc.). Overall, our findings show that (1) N1 synergizes with R848 in activating human and mouse DCs and (2) the synergistic effect based on various intracellular signaling events culminated in the activation of multiple transcriptional factors. These findings have important implications for future clinical trials since N1 and R848 synergistically promoted optimal Th1 lineage immune responses resulting in tumor rejection in mice.
Keywords: HMGN1; IFN; MAPK; NF-κB; R848; TLR; Th1 polarization; dendritic cell.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

