Using Dendritic Cell-Based Immunotherapy to Treat HIV: How Can This Strategy be Improved?
- PMID: 30619346
- PMCID: PMC6305438
- DOI: 10.3389/fimmu.2018.02993
Using Dendritic Cell-Based Immunotherapy to Treat HIV: How Can This Strategy be Improved?
Abstract
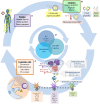
Harnessing dendritic cells (DC) to treat HIV infection is considered a key strategy to improve anti-HIV treatment and promote the discovery of functional or sterilizing cures. Although this strategy represents a promising approach, the results of currently published trials suggest that opportunities to optimize its performance still exist. In addition to the genetic and clinical characteristics of patients, the efficacy of DC-based immunotherapy depends on the quality of the vaccine product, which is composed of precursor-derived DC and an antigen for pulsing. Here, we focus on some factors that can interfere with vaccine production and should thus be considered to improve DC-based immunotherapy for HIV infection.
Keywords: HIV; clinical trial; dendritic cells; immunotherapy; therapeutic vaccine.
Figures
References
-
- Dalod M, Harzic M, Pellegrin I, Dumon B, Hoen B, Sereni D, et al. . Evolution of cytotoxic T lymphocyte responses to human immunodeficiency virus type 1 in patients with symptomatic primary infection receiving antiretroviral triple therapy. J Infect Dis. (1998) 178:61–69. 10.1086/515587 - DOI - PubMed
-
- Iglesias-Ussel MD, Romerio F. HIV reservoirs: the new frontier. AIDS Rev. (2011) 13:13–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

