Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment
- PMID: 30619378
- PMCID: PMC6306491
- DOI: 10.3389/fimmu.2018.03059
Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment
Abstract
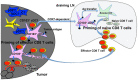
Dendritic cells (DCs) play a central role in the regulation of the balance between CD8 T cell immunity vs. tolerance to tumor antigens. Cross-priming, a process which DCs activate CD8 T cells by cross-presenting exogenous antigens, plays a critical role in generating anti-tumor CD8 T cell immunity. However, there are compelling evidences now that the tumor microenvironment (TME)-mediated suppression and modulation of tumor-infiltrated DCs (TIDCs) impair their function in initiating potent anti-tumor immunity and even promote tumor progression. Thus, DC-mediated cross-presentation of tumor antigens in tumor-bearing hosts often induces T cell tolerance instead of immunity. As tumor-induced immunosuppression remains one of the major hurdles for cancer immunotherapy, understanding how DCs regulate anti-tumor CD8 T cell immunity in particular within TME has been under intensive investigation. Recent reports on the Batf3-dependent type 1 conventional DCs (cDC1s) in anti-tumor immunity have greatly advanced our understanding on the interplay of DCs and CD8 T cells in the TME, highlighted by the critical role of CD103+ cDC1s in the cross-priming of tumor antigen-specific CD8 T cells. In this review, we will discuss recent advances in anti-tumor CD8 T cell cross-priming by CD103+ cDC1s in TME, and share perspective on future directions including therapeutic applications and memory CD8 T cell responses.
Keywords: CD103+ cDC1s; CD8 T cell immunity; anti-tumor immunity; cancer immunotherapy; cross-priming; tumor microenvironment.
Figures
References
-
- Slingluff CL, Jr, Cox AL, Stover JM, Jr, Moore MM, Hunt DF, Engelhard VH. Cytotoxic T-lymphocyte response to autologous human squamous cell cancer of the lung: epitope reconstitution with peptides extracted from HLA-Aw68. Cancer Res. (1994) 54:2731–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

