Waterlogging of Winter Crops at Early and Late Stages: Impacts on Leaf Physiology, Growth and Yield
- PMID: 30619425
- PMCID: PMC6306497
- DOI: 10.3389/fpls.2018.01863
Waterlogging of Winter Crops at Early and Late Stages: Impacts on Leaf Physiology, Growth and Yield
Erratum in
-
Corrigendum: Waterlogging of Winter Crops at Early and Late Stages: Impacts on Leaf Physiology, Growth and Yield.Front Plant Sci. 2020 Feb 4;10:1806. doi: 10.3389/fpls.2019.01806. eCollection 2019. Front Plant Sci. 2020. PMID: 32117354 Free PMC article.
Abstract
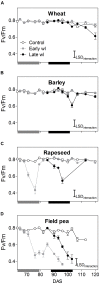
Waterlogging is expected to increase as a consequence of global climate change, constraining crop production in various parts of the world. This study assessed tolerance to 14-days of early- or late-stage waterlogging of the major winter crops wheat, barley, rapeseed and field pea. Aerenchyma formation in adventitious roots, leaf physiological parameters (net photosynthesis, stomatal and mesophyll conductances, chlorophyll fluorescence), shoot and root growth during and after waterlogging, and seed production were evaluated. Wheat produced adventitious roots with 20-22% of aerenchyma, photosynthesis was maintained during waterlogging, and seed production was 86 and 71% of controls for early- and late-waterlogging events. In barley and rapeseed, plants were less affected by early- than by late-waterlogging. Barley adventitious roots contained 19% aerenchyma, whereas rapeseed did not form aerenchyma. In barley, photosynthesis was reduced during early-waterlogging mainly by stomatal limitations, and by non-stomatal constraints (lower mesophyll conductance and damage to photosynthetic apparatus as revealed by chlorophyll fluorescence) during late-waterlogging. In rapeseed, photosynthesis was mostly reduced by non-stomatal limitations during early- and late-waterlogging, which also impacted shoot and root growth. Early-waterlogged plants of both barley and rapeseed were able to recover in growth upon drainage, and seed production reached ca. 79-85% of the controls, while late-waterlogged plants only attained 26-32% in seed production. Field pea showed no ability to develop root aerenchyma when waterlogged, and its photosynthesis (and stomatal and mesophyll conductances) was rapidly decreased by the stress. Consequently, waterlogging drastically reduced field pea seed production to 6% of controls both at early- and late-stages with plants being unable to resume growth upon drainage. In conclusion, wheat generates a set of adaptive responses to withstand 14 days of waterlogging, barley and rapeseed can still produce significant yield if transiently waterlogged during early plant stages but are more adversely impacted at the late stage, and field pea is not suitable for areas prone to waterlogging events of 14 days at either growth stage.
Keywords: aerenchyma; crops; photosynthesis; waterlogging; yield.
Figures



References
-
- Araki H., Hamada A., Hossain M. A., Takahashi T. (2012). Waterlogging at jointing and/or after anthesis in wheat induces early leaf senescence and impairs grain filling. Field Crops Res. 137 27–36. 10.1016/j.fcr.2012.09.006 - DOI
-
- Armstrong W. (1979). Aeration in higher plants. Adv. Bot. Res. 7 225–332. 10.1016/S0065-2296(08)60089-0 - DOI
-
- Ashraf M. A. (2012). Waterlogging stress in plants: a review. Afr. J. Agric. Res. 7 1976–1981. 10.5897/AJAR2016.12050 - DOI
-
- Barbour M. M., Warren C. R., Farquhar G. D., Forrester G. U. Y., Brown H. (2010). Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotope discrimination. Plant Cell Environ. 33 1176–1185. 10.1111/j.1365-3040.2010.02138.x - DOI - PubMed
-
- Belford R. K., Cannell R. Q., Thomson R. J., Dennis C. W. (1980). Effects of waterlogging at different stages of development on the growth and yield of peas (Pisumsativum L.). J. Sci. Food Agric. 31 857–869. 10.1002/jsfa.2740310902 - DOI
LinkOut - more resources
Full Text Sources

