Revisiting the Soybean GmNAC Superfamily
- PMID: 30619426
- PMCID: PMC6305603
- DOI: 10.3389/fpls.2018.01864
Revisiting the Soybean GmNAC Superfamily
Abstract
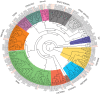
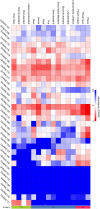
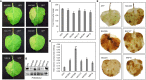
The NAC (NAM, ATAF, and CUC) genes encode transcription factors involved with the control of plant morph-physiology and stress responses. The release of the last soybean (Glycine max) genome assembly (Wm82.a2.v1) raised the possibility that new NAC genes would be present in the soybean genome. Here, we interrogated the last version of the soybean genome against a conserved NAC domain structure. Our analysis identified 32 putative novel NAC genes, updating the superfamily to 180 gene members. We also organized the genes in 15 phylogenetic subfamilies, which showed a perfect correlation among sequence conservation, expression profile, and function of orthologous Arabidopsis thaliana genes and NAC soybean genes. To validate our in silico analyses, we monitored the stress-mediated gene expression profiles of eight new NAC-genes by qRT-PCR and monitored the GmNAC senescence-associated genes by RNA-seq. Among ER stress, osmotic stress and salicylic acid treatment, all the novel tested GmNAC genes responded to at least one type of stress, displaying a complex expression profile under different kinetics and extension of the response. Furthermore, we showed that 40% of the GmNACs were differentially regulated by natural leaf senescence, including eight (8) newly identified GmNACs. The developmental and stress-responsive expression profiles of the novel NAC genes fitted perfectly with their phylogenetic subfamily. Finally, we examined two uncharacterized senescence-associated proteins, GmNAC065 and GmNAC085, and a novel, previously unidentified, NAC protein, GmNAC177, and showed that they are nuclear localized, and except for GmNAC065, they display transactivation activity in yeast. Consistent with a role in leaf senescence, transient expression of GmNAC065 and GmNAC085 induces the appearance of hallmarks of leaf senescence, including chlorophyll loss, leaf yellowing, lipid peroxidation and accumulation of H2O2. GmNAC177 was clustered to an uncharacterized subfamily but in close proximity to the TIP subfamily. Accordingly, it was rapidly induced by ER stress and by salicylic acid under late kinetic response and promoted cell death in planta. Collectively, our data further substantiated the notion that the GmNAC genes display functional and expression profiles consistent with their phylogenetic relatedness and established a complete framework of the soybean NAC superfamily as a foundation for future analyses.
Keywords: GmNAC superfamily; NAC; genome-wide expression profiling; phylogenetic analysis; senescence-associated genes; soybean; transcriptional factors.
Figures







References
-
- Block A., Toruño T. Y., Elowsky C. G., Zhang C., Steinbrenner J., Beynon J., et al. . (2014). The Pseudomonas syringae type III effector HopD1 suppresses effector-triggered immunity, localizes to the endoplasmic reticulum, and targets the Arabidopsis transcription factor NTL9. New Phytol. 201, 1358–1370. 10.1111/nph.12626 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

