Making sense of intralocus and interlocus sexual conflict
- PMID: 30619603
- PMCID: PMC6309128
- DOI: 10.1002/ece3.4629
Making sense of intralocus and interlocus sexual conflict
Abstract
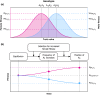
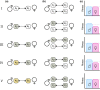
Sexual conflict occurs because males and females are exposed to different selection pressures. This can affect many aspects of female and male biology, such as physiology, behavior, genetics, and even population ecology. Its broad impact has caused widespread interest in sexual conflict. However, a key aspect of sexual conflict is often confused; it comprises two distinct forms: intralocus and interlocus sexual conflict (IASC and IRSC). Although both are caused by sex differences in selection, they operate via different proximate and ultimate mechanisms. Intralocus sexual conflict and IRSC are often not clearly defined as separate processes in the scientific literature, which impedes a proper understanding of each form as well as of their relative impact on sexual conflict. Furthermore, our current knowledge of the genetics of these phenomena is severely limited. This prevents us from empirically testing numerous theories regarding the role of these two forms of sexual conflict in evolution. Here, we clarify the distinction between IASC and IRSC, by discussing how male and female interests differ, how and when sex-specific adaptation occurs, and how this may lead to evolutionary change. We then describe a framework for their study, focusing on how future experiments may help identify the genetics underlying these phenomena. Through this, we hope to promote a more critical reflection on IASC and IRSC as well as underline the necessity of genetic and mechanistic studies of these two phenomena.
Keywords: natural selection; sexual antagonism; sexual selection; sexually antagonistic coevolution; sex‐specific adaptation.
Figures

References
-
- Arnqvist, G. , & Rowe, L. (2005). Sexual conflict. Princeton, NJ: Princeton University Press; 10.1515/9781400850600 - DOI
Publication types
LinkOut - more resources
Full Text Sources

