Electrochemical and Photoelectrochemical Properties of Nickel Oxide (NiO) With Nanostructured Morphology for Photoconversion Applications
- PMID: 30619811
- PMCID: PMC6299045
- DOI: 10.3389/fchem.2018.00601
Electrochemical and Photoelectrochemical Properties of Nickel Oxide (NiO) With Nanostructured Morphology for Photoconversion Applications
Abstract
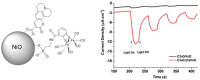
The cost-effective production of chemicals in electrolytic cells and the conversion of the radiation energy into electrical energy in photoelectrochemical cells (PECs) require the use of electrodes with large surface area, which possess either electrocatalytic or photoelectrocatalytic properties. In this context nanostructured semiconductors are electrodic materials of great relevance because of the possibility of varying their photoelectrocatalytic properties in a controlled fashion via doping, dye-sensitization or modification of the conditions of deposition. Among semiconductors for electrolysers and PECs the class of the transition metal oxides (TMOs) with a particular focus on NiO interests for the chemical-physical inertness in ambient conditions and the intrinsic electroactivity in the solid state. The latter aspect implies the existence of capacitive properties in TMO and NiO electrodes which thus act as charge storage systems. After a comparative analysis of the (photo)electrochemical properties of nanostructured TMO electrodes in the configuration of thin film the use of NiO and analogs for the specific applications of water photoelectrolysis and, secondly, photoelectrochemical conversion of carbon dioxide will be discussed.
Keywords: metal oxide nanostructures; nickel oxide nanoparticle; photoelectrochemical cells; photoelectrochemistry; semiconductor nanostructures; solar energy conversion.
Figures
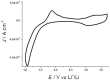
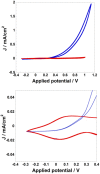
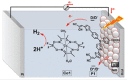
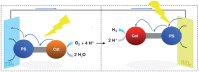


References
-
- Antila L. J., Ghamgosar P., Maji S., Tian H., Ott S., Hammarström L. (2016). Dynamics and photochemical H 2 evolution of Dye–NiO photocathodes with a biomimetic FeFe-catalyst. ACS Energy Lett. 1, 1106–1111. 10.1021/acsenergylett.6b00506 - DOI
-
- Armstrong N. R., Lin A. W. C., Fujihira M., Kuwana T. (1976). Electrochemical and surface characteristics of tin oxide and indium oxide electrodes. Anal. Chem. 48, 741–750. 10.1021/ac60368a035 - DOI
-
- Awais M., Dini D., Don MacElroy J. M., Halpin Y., Vos J. G., Dowling D. P. (2013a). Electrochemical characterization of NiO electrodes deposited via a scalable powder microblasting technique. J. Electroanal. Chem. 689, 185–192. 10.1016/j.jelechem.2012.11.025 - DOI
Publication types
LinkOut - more resources
Full Text Sources

