D-3-Phosphoglycerate Dehydrogenase
- PMID: 30619878
- PMCID: PMC6300728
- DOI: 10.3389/fmolb.2018.00110
D-3-Phosphoglycerate Dehydrogenase
Abstract
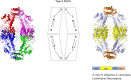
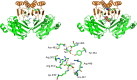
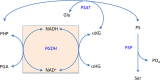
l-Serine is the immediate precursor of d-serine, a major agonist of the N-methyl-d-aspartate (NMDA) receptor. l-Serine is a pivotal amino acid since it serves as a precursor to a large number of essential metabolites besides d-serine. In all non-photosynthetic organisms, including mammals, a major source of l-serine is the phosphorylated pathway of l-serine biosynthesis. The pathway consists of three enzymes, d-3-phosphoglycerate dehydrogenase (PGDH), phosphoserine amino transferase (PSAT), and l-phosphoserine phosphatase (PSP). PGDH catalyzes the first step in the pathway by converting d-3-phosphoglycerate (PGA), an intermediate in glycolysis, to phosphohydroxypyruvate (PHP) concomitant with the reduction of NAD+. In some, but not all organisms, the catalytic activity of PGDH can be regulated by feedback inhibition by l-serine. Three types of PGDH can be distinguished based on their domain structure. Type III PGDHs contain only a nucleotide binding and substrate binding domain. Type II PGDHs contain an additional regulatory domain (ACT domain), and Type I PGDHs contain a fourth domain, termed the ASB domain. There is no consistent pattern of domain content that correlates with organism type, and even when additional domains are present, they are not always functional. PGDH deficiency results in metabolic defects of the nervous system whose systems range from microcephaly at birth, seizures, and psychomotor retardation. Although deficiency of any of the pathway enzymes have similar outcomes, PGDH deficiency is predominant. Dietary or intravenous supplementation with l-serine is effective in controlling seizures but has little effect on psychomotor development. An increase in PGDH levels, due to overexpression, is also associated with a wide array of cancers. In culture, PGDH is required for tumor cell proliferation, but extracellular l-serine is not able to support cell proliferation. This has led to the hypothesis that the pathway is performing some function related to tumor growth other than supplying l-serine. The most well-studied PGDHs are bacterial, primarily from Escherichia coli and Mycobacterium tuberculosis, perhaps because they have been of most interest mechanistically. However, the relatively recent association of PGDH with neuronal defects and human cancers has provoked renewed interest in human PGDH.
Keywords: biosynthesis; d-serine; dehydrogenase; l-serine; phosphoglycerate.
Figures
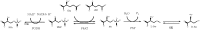




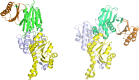


References
-
- Ali V., Hashimoto T., Shigeta Y., Nozaki T. (2004). Molecular and biochemical characterization of d-phosphoglycerate dehydrogenase from Entamoeba histolytica: a unique enteric protozoan parasite that possesses both phosphorylated and nonphosphorylated serine metabolic pathways. Eur. J. Biochem. 271, 2670–2681. 10.1111/j.1432-1033.2004.04195.x - DOI - PubMed
-
- Al-Rabiee R., Lee E. J., Grant G. A. (1996a). The mechanism of velocity modulated allosteric regulation in d-3-Phosphoglycerate dehydrogenase: cross-linking adjacent regulatory domains with engineered disulfides mimics effector binding. J. Biol. Chem. 271, 13013–13017. 10.1074/jbc.271.22.13013 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

