Why the Energy Landscape of Barnase Is Hierarchical
- PMID: 30619881
- PMCID: PMC6306431
- DOI: 10.3389/fmolb.2018.00115
Why the Energy Landscape of Barnase Is Hierarchical
Abstract
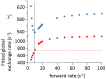
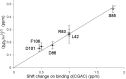
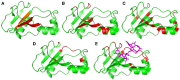
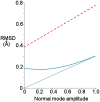
We have used NMR and computational methods to characterize the dynamics of the ribonuclease barnase over a wide range of timescales in free and inhibitor-bound states. Using temperature- and denaturant-dependent measurements of chemical shift, we show that barnase undergoes frequent and highly populated hinge bending. Using relaxation dispersion, we characterize a slower and less populated motion with a rate of 750 ± 200 s-1, involving residues around the lip of the active site, which occurs in both free and bound states and therefore suggests conformational selection. Normal mode calculations characterize correlated hinge bending motions on a very rapid timescale. These three measurements are combined with previous measurements and molecular dynamics calculations on barnase to characterize its dynamic landscape on timescales from picoseconds to milliseconds and length scales from 0.1 to 2.5 nm. We show that barnase has two different large-scale fluctuations: one on a timescale of 10-9-10-6 s that has no free energy barrier and is a hinge bending that is determined by the architecture of the protein; and one on a timescale of milliseconds (i.e., 750 s-1) that has a significant free energy barrier and starts from a partially hinge-bent conformation. These two motions can be described as hierarchical, in that the more highly populated faster motion provides a platform for the slower (less probable) motion. The implications are discussed. The use of temperature and denaturant is suggested as a simple and general way to characterize motions on the intermediate ns-μs timescale.
Keywords: biophysics; conformational selection; molecular dynamics; nuclear magnetic resonance (NMR); protein dynamics; relaxation dispersion; structural biology.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

