Recent advances of on-demand dissolution of hydrogel dressings
- PMID: 30619904
- PMCID: PMC6310937
- DOI: 10.1186/s41038-018-0138-8
Recent advances of on-demand dissolution of hydrogel dressings
Abstract
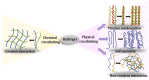
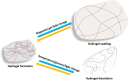
Wound management is a major global challenge and a big financial burden to the healthcare system due to the rapid growth of chronic diseases including the diabetes, obesity, and aging population. Modern solutions to wound management include hydrogels that dissolve on demand, and the development of such hydrogels is of keen research interest. The formation and subsequent on-demand dissolution of hydrogels is of keen interest to scientists and clinicians. These hydrogels have excellent properties such as tissue adhesion, swelling, and water absorption. In addition, these hydrogels have a distinctive capacity to form in situ and dissolve on-demand via physical or chemical reactions. Some of these hydrogels have been successfully used as a dressing to reduce bleeding in hepatic and aortal models, and the hydrogels remove easily afterwards. However, there is an extremely wide array of different ways to synthesize these hydrogels. Therefore, we summarize here the recent advances of hydrogels that dissolve on demand, covering both chemical cross-linking cases and physical cross-linking cases. We believe that continuous exploration of dissolution strategies will uncover new mechanisms of dissolution and extend the range of applications for hydrogel dressings.
Keywords: Hydrogel; On-demand dissolution; Wound dressing; Wound management.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.
Figures




Similar articles
-
On-Demand Dissolution of Chemically Cross-Linked Hydrogels.Acc Chem Res. 2017 Feb 21;50(2):151-160. doi: 10.1021/acs.accounts.6b00547. Epub 2017 Feb 7. Acc Chem Res. 2017. PMID: 28170219 Free PMC article.
-
Hyaluronic Acid/Chondroitin Sulfate-Based Dynamic Thiol-Aldehyde Addition Hydrogel: An Injectable, Self-Healing, On-Demand Dissolution Wound Dressing.Materials (Basel). 2024 Jun 19;17(12):3003. doi: 10.3390/ma17123003. Materials (Basel). 2024. PMID: 38930372 Free PMC article.
-
Transparent Conductive Supramolecular Hydrogels with Stimuli-Responsive Properties for On-Demand Dissolvable Diabetic Foot Wound Dressings.Macromol Rapid Commun. 2020 Dec;41(24):e2000441. doi: 10.1002/marc.202000441. Epub 2020 Oct 21. Macromol Rapid Commun. 2020. PMID: 33089609 Review.
-
In situ gelling and dissolvable hydrogels for use as on-demand wound dressings for burns.Biomater Sci. 2021 Oct 12;9(20):6842-6850. doi: 10.1039/d1bm00711d. Biomater Sci. 2021. PMID: 34486599 Free PMC article.
-
Research status of self-healing hydrogel for wound management: A review.Int J Biol Macromol. 2020 Dec 1;164:2108-2123. doi: 10.1016/j.ijbiomac.2020.08.109. Epub 2020 Aug 13. Int J Biol Macromol. 2020. PMID: 32798548 Review.
Cited by
-
A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery.Antibiotics (Basel). 2020 Sep 28;9(10):648. doi: 10.3390/antibiotics9100648. Antibiotics (Basel). 2020. PMID: 32998197 Free PMC article. Review.
-
Cohesion mechanisms for bioadhesives.Bioact Mater. 2021 Nov 11;13:105-118. doi: 10.1016/j.bioactmat.2021.11.008. eCollection 2022 Jul. Bioact Mater. 2021. PMID: 35224295 Free PMC article. Review.
-
Growth Factor and Cytokine Delivery Systems for Wound Healing.Cold Spring Harb Perspect Biol. 2022 Aug 1;14(8):a041234. doi: 10.1101/cshperspect.a041234. Cold Spring Harb Perspect Biol. 2022. PMID: 35667794 Free PMC article. Review.
-
Nanomaterials for Wound Dressings: An Up-to-Date Overview.Molecules. 2020 Jun 10;25(11):2699. doi: 10.3390/molecules25112699. Molecules. 2020. PMID: 32532089 Free PMC article. Review.
-
Glutathione Therapy in Diseases: Challenges and Potential Solutions for Therapeutic Advancement.Curr Mol Med. 2024;24(10):1219-1230. doi: 10.2174/1566524023666230818142831. Curr Mol Med. 2024. PMID: 37594114 Review.
References
-
- Noor S, Zubair M, Ahmad J. Diabetic foot ulcer: A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. 2015;3:192–9. - PubMed
-
- Liu X, Chen B, Xiaoling R, Liao Q, Xie W, Zhang Y, et al. The practice of standardized management in wound/stoma care clinic. J Nurs Sci. 2017;22:41–43.
Publication types
LinkOut - more resources
Full Text Sources

