Resolution of ascites and hepatic encephalopathy and absence of variceal bleeding in decompensated hepatitis C virus cirrhosis patients
- PMID: 30619944
- PMCID: PMC6308043
- DOI: 10.1002/jgh3.12091
Resolution of ascites and hepatic encephalopathy and absence of variceal bleeding in decompensated hepatitis C virus cirrhosis patients
Abstract
Background and aims: The aims of this study were to examine changes in the proportion of decompensated hepatitis C virus (HCV) cirrhosis patients with ascites, hepatic encephalopathy, and variceal bleeding at pretreatment compared to 3 and 12 months post-sustained virological response (SVR) and to compare pretreatment and post-SVR model of end-stage liver disease and Child-Pugh scores and alpha-fetoprotein levels.
Methods: Electronic medical records of 64 decompensated HCV cirrhosis patients who received direct-acting antivirals were reviewed. The McNemar-Bowker test and the Wilcoxon-Signed Rank test were used to compare patient outcomes.
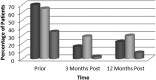
Results: Ascites was resolved in 29% of patients 3 months post-SVR (65% vs 36%, P < 0.01) and in 35% of patients 12 months post-SVR (65% vs 30%, P = 0.07). Hepatic encephalopathy was resolved in 54% of patients 3 months post-SVR (70% vs 16%, P < 0.01) and in 48% of patients 12 months post-SVR (70% vs 22% P = 0.03). Variceal bleeding was absent in 32% of patients 3 months post-SVR (35% vs 3%, P < 0.01) and in 27% of patients 12 months post-SVR (35% vs 8%, P < 0.01). Alpha-fetoprotein levels were significantly reduced post-SVR, but model of end-stage liver disease and Child-Pugh scores were not.
Conclusions: Decompensated HCV cirrhosis patients who achieved SVR with direct-acting antiviral treatment had significant reductions in manifestations of hepatic decompensation sustainable up to 1 year post-SVR.
Keywords: decompensated cirrhosis; direct acting antivirals; hepatitis C; treatment.
Figures
References
-
- Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014; 61: S45–57. - PubMed
-
- Dore GJ, Ward J, Thursz M. Hepatitis C disease burden and strategies to manage the burden (Guest Editors Mark Thursz, Gregory Dore and John Ward). J. Viral Hepat. 2014; 21: 1–4. - PubMed
-
- Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology. 2013; 57: 1333–42. - PubMed
-
- Davis GL, Alter MJ, El‐Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)‐infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010; 138: 513–21. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

 , HE;
, HE;  Ascites;
Ascites;  , VB.
, VB.
