Neuroprotective role of vitamin D in primary neuronal cortical culture
- PMID: 30619951
- PMCID: PMC6312860
- DOI: 10.1016/j.ensci.2018.12.004
Neuroprotective role of vitamin D in primary neuronal cortical culture
Abstract
Background: A role of Vitamin D in brain development and function has been gaining support over the last decade. There are compelling pieces of evidence that suggest vitamin D may have a neuroprotective role. The administration of vitamin D or its metabolites has been shown to reduce neurological injury and/or neurotoxicity in a variety of animal systems. The detail biochemical mechanism mediating neurons, to its ability to withstand greater oxidative stress in the presence of Vitamin D is unclear. This study was undertaken to study the biochemical effect of treatments of primary cortical neuronal cultures, with the active form of vitamin D(1,25(OH)2D3), against the induced oxidative stress.
Methods: Primary neuronal cultures from cerebral cortex were set up from neonatal (from 6 to 7 days old) Wister Rat's brain. Different doses of [1,25(OH)2D3], ranges from 0 to 1 μg/ml, was added to the culture medium and the cells were cultured in its presence for 24 h to 120 h. The effect of induced extracellular oxidative stress was measured by subjecting these cultured cells with 0.5 mM H2O2 for 2 h, prior to collection of condition medium and the cell pellet for biochemical assay. The control and H2O2 treated cultures were maintained in similar culture conditions, for similar periods of time without any [1,25(OH)2D3] treatments.
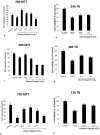
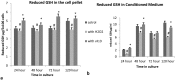
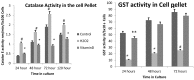
Result: The optimum concentration of [1,25(OH)2D3] for treatment of primary cortical neuronal cultures was found to be 0.25 μg/ml by Trypan exclusion assay and MTT assay. Pre-treatments of cultured neuronal cells with 0.25 μg/ml of [1,25(OH)2D3] caused significantly increased levels of reduced glutathione, accompanied by a similar increase in the enzyme levels of GST, to neutralize the induced oxidative stress by H2O2. The level of Lipid peroxidation was significantly higher in the cells treated with H2O2 alone, but it was completely reversed in the neuronal cultures pre-treated with [1,25(OH)2D3]. The levels of Catalase enzyme also significantly reduced (≥0.05) in the [1,25(OH)2D3] pre-treated neuronal cultures.
Conclusion: We concluded that the systemic treatment of primary neuronal cultures with [1,25(OH)2D3] gave better protection to neurons against the induced oxidative stress, as shown by quantitative measurements of various biomarkers of oxidative stress. This study also suggested that Vitamin D is vital for the growth, survival, and proliferation of the neurons and hence it has a potential therapeutic role against various neurodegenerative diseases.
Figures

References
-
- Cui X., Gooch H., Groves N.J. Vitamin D and the brain: key questions for future research. J. Steroid Biochem. Mol. Biol. 2015;148:305–309. - PubMed
-
- Bains J.S., Shaw C.A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Brain Res. Rev. 1997;25(3):335–358. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

