Hepatitis B Treatment: What We Know Now and What Remains to Be Researched
- PMID: 30619990
- PMCID: PMC6312657
- DOI: 10.1002/hep4.1281
Hepatitis B Treatment: What We Know Now and What Remains to Be Researched
Abstract
Chronic hepatitis B virus (HBV) infection remains a major global health burden. Currently, two types of treatment, interferons (IFNs) and nucleos(t)ide analogues (NAs), have been approved. These treatments are effective in suppressing HBV replication and in decreasing the risk of developing cirrhosis, liver failure, hepatocellular carcinoma (HCC), and death. However, these treatments do not eliminate the virus, and the risk of HCC remains. This review article summarizes current knowledge about the safety, efficacy, and clinical indications of hepatitis B treatment. It also discusses limitations of existing treatment, gaps in knowledge, and feasibility of a hepatitis B cure.
Figures
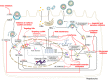

References
-
- Polaris Observatory Collaborators . Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383‐403. - PubMed
-
- Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta‐analysis. Hepatology 2016;63:284‐306. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

